Percent Composition
advertisement

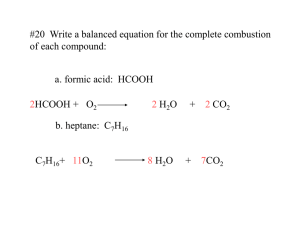
The chemical composition can be expressed as the mass percent of each element in the compound. Carry out % mass to the hundredths place. Example: Determine the percent composition of C3H8. Assume you have one mole of the substance. 3 C = 3(12.01) = 36.03 g %C 36.03g 100 81.68% 44.11g 8 H = 8(1.01) = 8.08 g %H 8.08g 100 18.32% 44.11g Total MW = 44.11 g Example: Determine the percent composition of iron(III) sulfate. Iron(III) sulfate = Fe2(SO4)3 2 Fe = 2(55.85) = 111.70 g 3 S = 3(32.07) = 96.21 g 12 O = 12(16.00) = 192.00 g Total MW = 399.91 g %Fe 111.70g 100 27.93% 399.91g %S 96.21g 100 24.06% 399.91g %O 192.00g 100 48.01% 399.91g Hydrated Compounds Some compounds exist in a “hydrated” state. Some specific number of water molecules are present for each molecule of the compound. Example: oxalic acid (COOH)2 can be obtained in the laboratory as (COOH)2•2H2O. Note: the dot in (COOH)2•2H2O shows that the crystals of oxalic acid contain 2 water molecules per (COOH)2 molecule. Example: oxalic acid (COOH)2 can be obtained in the laboratory as (COOH)2•2H2O. The molar mass of (COOH)2 = 90.04 g/mol 90.04 % anhydrous molecule 100 71.41% 126 .08 The molar mass of (COOH)2•2H2O = 126.08 g/mol 36.04 % water 100 28.59% 126 .08 Water can be driven out of a hydrated compound by heating it to leave an “anhydrous” (without water) compound. Example: A 7.0 g sample of calcium nitrate, Ca(NO3) 2•4H2O, is heated to constant mass. How much anhydrous salt remains? % mass anhydrous salt = 164.10g 100 69.48% 236.18g Mass of anhydrous salt remaining = .6948 7.0g 4.9 g Hydration Number Some molecules attach themselves to water molecules. This is done in set numbers, depending on the molecule. For example, Magnesium sulfate attaches to 7 water molecules. We say it’s hydration number is 7 MgSO47H2O (Magnesium Sulfate Heptahydrate) Anhydrides A compound that is normally a hydrate and has lost its hydration water is said to be anhydrous and is called an anhydride. BaCl22H2O Barium Chloride Dihydrate BaCl2 Barium Chloride Anhydride -or- Anhydrous Barium Chloride Finding the Hydration Number The hydration number can be conveniently found by heating the compound and measuring its mass loss. This mass loss is usually due to the hydration water molecules being driven off. For example… A 15.35 g sample of Strontium nitrate, Sr(NO3)2nH2O, is heated to a constant mass of 11.45 g. Calculate the hydration number. Sample Data: Mass Hydrate 15.35g Mass Anhydride 11.45g Mass of Water (mass loss) 3.90g Calculations Moles Anhydride 11.45g Sr(NO3 ) 2 1 molSr(NO3 ) 2 0.0541molSr(NO3 ) 2 211.64g Sr(NO3 ) 2 Moles Water 3.90g H 2O 1 mol H 2O 0.216 mol H 2O 18.02g H 2O Molar ratio… mol H O 0.216 H 2O 2 3.99 4 molSr(NO3 ) 2 0.0541molSr(NO3 ) 2 Hydration Number is 4, Sr(NO3)24H2O (Strontium Nitrate Tetrahydrate)