Lecture 33
advertisement

Lon-Capa 8th (final) HW assignment due Tuesday, December 2 by 5 pm. Quiz #6 opens today at 5 pm. Due Tuesday, December 2 by 10 pm. Exams and Practice Exams on website 1 Evaluating Mechanisms The rate law comes from the ratedetermining step (the “slow” step). Fast equilibrium. Rate forward = rate reverse. Rate law cannot include intermediates. Steady-state approximation. [intermediate] = constant. d[intermediate]/dt = 0 Rate law cannot include intermediates. 2 Clicker Question The reaction 2A + B C has the following proposed mechanism A+B D (fast equil.) D+BE (slow) E + A C + B (fast) The rate law from this mechanism is a) rate = k[A][B] d) rate = k[A][B]2 b) rate = k[A]2[B] e) I don’t know c) rate = k[A]2[B]2 d) rate = k[A][B]2 3 Reaction Profiles 4 Recall for st 1 Lecture 5 Transition State 6 Rate constant (k) vs. T 7 ln(k) vs. 1/T 8 Catalyzed Pathways Have a Lower Activation Energy 9 Catalyzed Pathways Have a Lower Activation Energy 10 Zero-order (catalyst) 11 Chapter 15: 115 Consider the hypothetical reaction: A + B + 2C 2D + 3E In a study of this reaction, three experiments were run at the same temperature. The rate is defined as –d[B]/dt. Expt 1: [A]0 = 2.0 M, [C]0 = 1.0 M, [B]0 = 1.0 x 10-3 M Write the rate law for this reaction, and calculate the rate constant. [B] Time (s) (mol/L) 2.7 x 10-4 1.0 x 105 1.6 x 10-4 2.0 x 105 1.1 x 10-4 3.0 x 105 8.5 x 10-5 4.0 x 105 6.9 x 10-5 5.0 x 105 5.8 x 10-5 6.0 x 105 12 Chapter 15: 115 Consider the hypothetical reaction: A + B + 2C 2D + 3E In a study of this reaction, three experiments were run at the same temperature. The rate is defined as –d[B]/dt. Expt 2: [B]0 = 3.0 M, [C]0 = 1.0 M, [A]0 = 1.0 x 10-2 M Write the rate law for this reaction, and calculate the rate constant. [A] Time (s) (mol/L) 8.9 x 10-3 1.0 7.1 x 10-3 3.0 5.5 x 10-3 5.0 3.8 x 10-3 8.0 2.9 x 10-3 10.0 2.0 x 10-3 13.0 13 Chapter 15: 115 Consider the hypothetical reaction: A + B + 2C 2D + 3E In a study of this reaction, three experiments were run at the same temperature. The rate is defined as –d[B]/dt. Expt 3: [A]0 = 10.0 M, [B]0 = 5.0 M, [C]0 = 5.0 x 10-1 M Write the rate law for this reaction, and calculate the rate constant. [C] (mol/L) 0.43 Time (s) 1.0 x 10-2 0.36 2.0 x 10-2 0.29 3.0 x 10-2 0.22 4.0 x 10-2 0.15 5.0 x 10-2 0.08 6.0 x 10-2 14








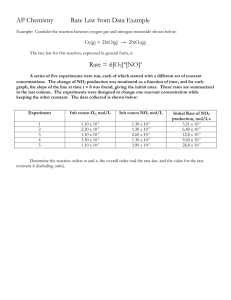
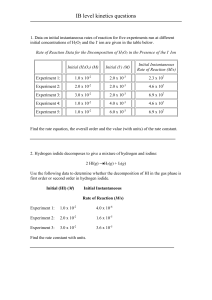

![Some Examples of Experimental Rate Laws Rate = k[A]](http://s3.studylib.net/store/data/008429765_1-55c81f2e6368c61bd42b7e157f3d34f1-300x300.png)