Uncertainty in Lab Measurements and Reading Volumes Purpose
advertisement

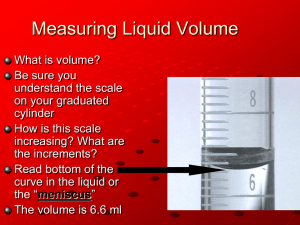
Uncertainty in Lab Measurements and Reading Volumes Purpose: To gain experience in measuring and recording length, mass, and volume using the proper number of significant figures and correct units. Discussion: All lab experiments fall under two broad types: qualitative and quantitative. In the qualitative lab, changes are studied but the amounts of each substance are not critical to the outcome of the lab. Quantitative labs depend on exact measurements of all substances and variations in the quantities used in the lab change the outcome of the experiment. Measurement is a very important part of the chemistry lab. The instruments used to measure differ in their design, their cost, and in their accuracy. Instruments are never exact. The term accuracy means how close the measurement is to the exact value. It should not be confused with the term precision. Precision means how close a value can be repeated. A student can be very precise, but still inaccurate with measurements. Significant figures are those that are actually measured as well as one additional estimate. The number of digits recorded in a specific measurement indicates the accuracy of the particular instrument used. It is essential that the correct number of significant figures be recorded with each instrument used so that a false impression of the accuracy is not implied in the recorded measurement. Data recorded in the lab has two parts: the significant figures and the unit for that measurement. You have probably reviewed the Metric system units. The most common units used in the lab depend on the equipment being used and are not necessarily the SI unit. They are: QUANTITY Mass Volume Length Temperature UNIT Gram Milliliter Centimeter Celsius degrees INSTRUMENT Balance Graduated cylinder, pipet Meter stick, metric ruler Thermometer The SI unit for volume is the cubic decimeter, dm3, or liter. The lab equipment that is used for measuring volume is marked off in milliliters, so that this unit is used in lab measurements. Other equipment such as beakers, Erlenmeyer flasks, are also marked off in milliliters. These are approximate volumes and are not used to record accurate volumes. In this lab various instruments will be used. These differ in their accuracy. You must read and record each measurement with the proper number of significant figures and the correct unit. The instrument with the greatest number of significant figures is the most accurate. When making any measurements in the lab always use the instrument that gives the greatest accuracy. Pre-lab Assignment: Answer the following pre-lab questions – 1. What is accuracy? 2. What is precision? 3. What is the difference between accuracy and precision? 4. What is uncertainty of a measurement? 5. How to determine the uncertainty of a measurement? 6. What are the two parts of every measurement? 7. In recording a measurement, is the first estimated digit considered significant? 8. A student records 38.976 as the mass of an object. What is wrong with this measurement? 9. How to properly read a ruler, graduated cylinder? DO NOT DISTURB THE POSITION OF ANY AMOUNT OF LIQUID AT ANY STATION. PART A: Uncertainty in Various Lab Equipment 1. Observe the 9 different instruments on the lab table. Record your measurements with the correct number of sig figs, and indicate the number of sig figs in Table 1. DATA: Table 1: ____________________________________________________ INSTRUMENT 10 mL Graduated Cylinder 25 mL Graduated Cylinder 100 mL Graduated Cylinder 250 mL Beaker Thermometer Electronic Balance Meter stick Metric ruler MEASUREMENT (specify the uncertainty for each of the tools) # OF SIG. FIGS. PART B: Reading Volumes- a Sig Fig Activity 1. Go to the other side of the lab table. 2. Each of the different volume instruments contains 5.0 mL of water. 3. Using the information from PART A, fill in Table 2 below. Table 2: ______________________________________________ INSTRUMENT VOLUME (mL) UNCERTAINTY (mL) # of SIG FIGS 100 mL Graduated Cylinder 25 mL Graduated Cylinder 10 mL Graduated Cylinder 100 mL Beaker 125 mL Erlenmeyer Flask POST-LAB QUESTIONS: 1. A square of metal foil has a 6.00-cm side. Calculate the area of the foil and record your answer in the correct number of sig figs. 2. Of all the instruments used in this lab to measure volume, which is: a) least accurate. Explain. b) most accurate. Explain. 3. If the actual mass of an object is 27.987 grams and you obtain the following data in lab, how would you describe your results in terms of accuracy and precision? 27.120 g 27.122 g 27.119 g 4. Which graduated cylinder is the least accurate? Explain your choice. 5. In Part B, are the measurements the same or different? What factors could account for the difference, if any? Which “reading“ was more precise? More accurate? (Note: Do we know the accuracy?) 6. If the volume of 5.0 mL of water was dispensed accurately into each type of volume instrument, rank the different types of glassware in order from most accurate to least accurate. 7. If you were asked to measure 21.4 mL of water, which instrument would be chosen to give the most accurate volume? 8. How many significant figures are in each of the following? a) 0.00130 g ________________________ b) 27.0090 g ________________________ c) 50.0 mL __________________________ d) 1.000g ___________________________ 9. The mass of an object is obtained on two types of balances. The values obtained are: #1 108.9 g #2 108.9270 g What are the uncertainties of each balance? Which is the more accurate balance? Why?