8th Science
advertisement
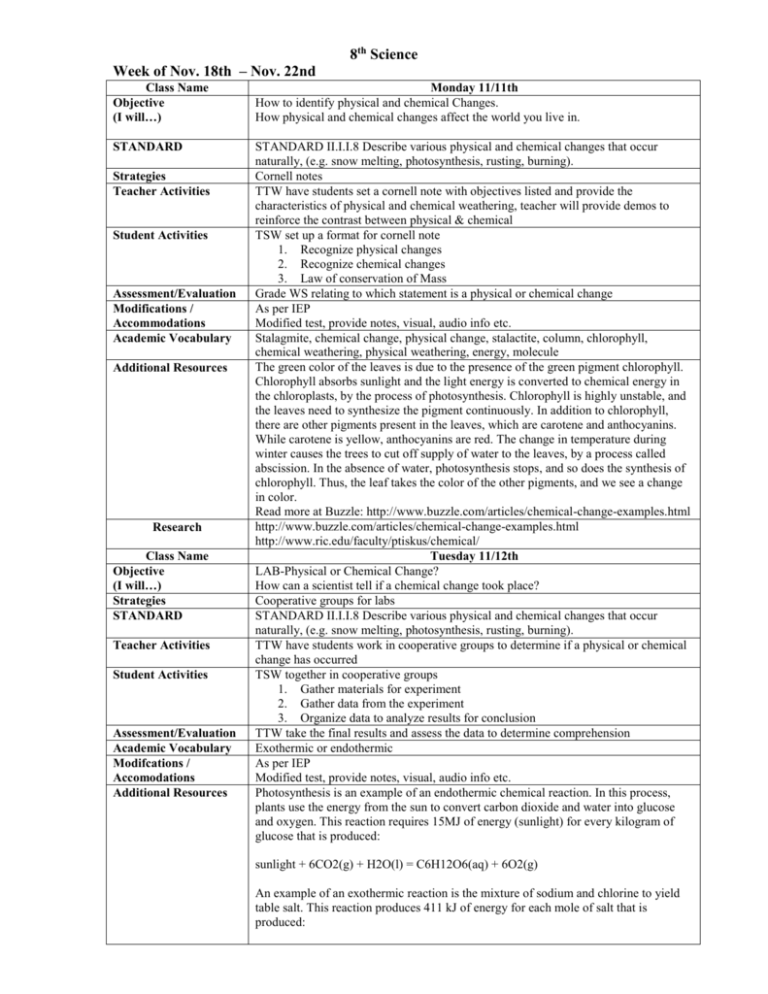
8th Science Week of Nov. 18th – Nov. 22nd Class Name Objective (I will…) Monday 11/11th How to identify physical and chemical Changes. How physical and chemical changes affect the world you live in. STANDARD STANDARD II.I.I.8 Describe various physical and chemical changes that occur naturally, (e.g. snow melting, photosynthesis, rusting, burning). Cornell notes TTW have students set a cornell note with objectives listed and provide the characteristics of physical and chemical weathering, teacher will provide demos to reinforce the contrast between physical & chemical TSW set up a format for cornell note 1. Recognize physical changes 2. Recognize chemical changes 3. Law of conservation of Mass Grade WS relating to which statement is a physical or chemical change As per IEP Modified test, provide notes, visual, audio info etc. Stalagmite, chemical change, physical change, stalactite, column, chlorophyll, chemical weathering, physical weathering, energy, molecule The green color of the leaves is due to the presence of the green pigment chlorophyll. Chlorophyll absorbs sunlight and the light energy is converted to chemical energy in the chloroplasts, by the process of photosynthesis. Chlorophyll is highly unstable, and the leaves need to synthesize the pigment continuously. In addition to chlorophyll, there are other pigments present in the leaves, which are carotene and anthocyanins. While carotene is yellow, anthocyanins are red. The change in temperature during winter causes the trees to cut off supply of water to the leaves, by a process called abscission. In the absence of water, photosynthesis stops, and so does the synthesis of chlorophyll. Thus, the leaf takes the color of the other pigments, and we see a change in color. Read more at Buzzle: http://www.buzzle.com/articles/chemical-change-examples.html http://www.buzzle.com/articles/chemical-change-examples.html http://www.ric.edu/faculty/ptiskus/chemical/ Tuesday 11/12th LAB-Physical or Chemical Change? How can a scientist tell if a chemical change took place? Cooperative groups for labs STANDARD II.I.I.8 Describe various physical and chemical changes that occur naturally, (e.g. snow melting, photosynthesis, rusting, burning). TTW have students work in cooperative groups to determine if a physical or chemical change has occurred TSW together in cooperative groups 1. Gather materials for experiment 2. Gather data from the experiment 3. Organize data to analyze results for conclusion TTW take the final results and assess the data to determine comprehension Exothermic or endothermic As per IEP Modified test, provide notes, visual, audio info etc. Photosynthesis is an example of an endothermic chemical reaction. In this process, plants use the energy from the sun to convert carbon dioxide and water into glucose and oxygen. This reaction requires 15MJ of energy (sunlight) for every kilogram of glucose that is produced: Strategies Teacher Activities Student Activities Assessment/Evaluation Modifications / Accommodations Academic Vocabulary Additional Resources Research Class Name Objective (I will…) Strategies STANDARD Teacher Activities Student Activities Assessment/Evaluation Academic Vocabulary Modifcations / Accomodations Additional Resources sunlight + 6CO2(g) + H2O(l) = C6H12O6(aq) + 6O2(g) An example of an exothermic reaction is the mixture of sodium and chlorine to yield table salt. This reaction produces 411 kJ of energy for each mole of salt that is produced: Research Class Name Objective (I will…) Strategies STANDARD Na(s) + 0.5Cl2(s) = NaCl(s) http://chemistry.about.com/cs/generalchemistry/a/aa051903a.htm Wednesday 11/13th Practice Writing Teacher Activities Recall, quick write STANDARD II.I.I.8 Describe various physical and chemical changes that occur naturally, (e.g. snow melting, photosynthesis , rusting, burning). TTW students writing prompts to practice for the SBA Student Activities Assessment/Evaluation Academic Vocabulary Modifcations / Accomodations Additional Resources Search Class Name Objective (I will…) TSW take the writing prompts and practice TT take a grade on content of the paragraph Brainstorming, Quickwrite, guidewriting, recall As per IEP Modified test, provide notes, visual, audio info etc. Questions related for writing prompt http://www.writingfix.com/WAC/Writing_Across_Curriculum_RAFTS_Science.htm Thursday 11/14th How to identify physical and chemical Changes. How physical and chemical changes affect the world you live in. STANDARD STANDARD II.I.I.8 Describe various physical and chemical changes that occur naturally, (e.g. snow melting, photosynthesis, rusting, burning). Recall, identify, interpret TTW have students use QQT for review, PPT review test questions over metric and physical & chemical changes TSW use the lab sheet and follow the instructions on the lab to determine if the change is a physical or chemical. Quiz over metric & physical / chemical changes Metric, physical & chemical changes As per IEP Modified test, provide notes, visual, audio info etc. PPT, QQT cards, http://chemistry.about.com/cs/generalchemistry/a/aa051903a.htm Strategies Teacher Activities Student Activities Assessment/Evaluation Academic Vocabulary Modifcations / Accomodations Additional Resources Research Class Name Objective (I will…) Strategies STANDARD Teacher Activities Student Activities Assessment/Evaluation Academic Vocabulary Modifcations / Accomodations Additional Resources Friday 11/15th Rates of Chemical Reactions How to measure the speed of a chemical reaction How chemical reactions can be speeded up or slowed down Guided reading STANDARD II.I.I.9 Identify factors that influence the rate at which chemical reactions of occur (e.g. temperature, concentration). TTW have students make a 12 square foldable and number their reading paragraphs and read with their cooperative group and answer questions in the 12 squares and the margins of their read and do. TSW make a 12 square foldable and number their squares in relation to their paragraph. Students will work in their cooperative groups. Teacher will spot check to monitor progress Activation energy, catalyst, concentration, enzyme, inhibitor, rate of reaction As per IEP Modified test, provide notes, visual, audio info etc. The rate of a reaction is the speed at which a reaction happens. If a reaction has a low rate, that means the molecules combine at a slower speed than a reaction with a high rate. There is another big idea for rates of reaction called collision theory. The collision theory says that as more collisions in a system occur, there will be more combinations of molecules bouncing into each other. If there are a higher number of collisions in a system, more combinations of molecules can occur. The reaction will go faster and the rate of that reaction will be higher. Even though they are both liquids, think about how slowly molecules move in honey when compared to your soda. There Research are a lower number of collisions in the honey http://www.chem4kids.com/files/react_rates.html