Unit: Atomic and Molecular Structure Unit Review Part B Key Part E
advertisement
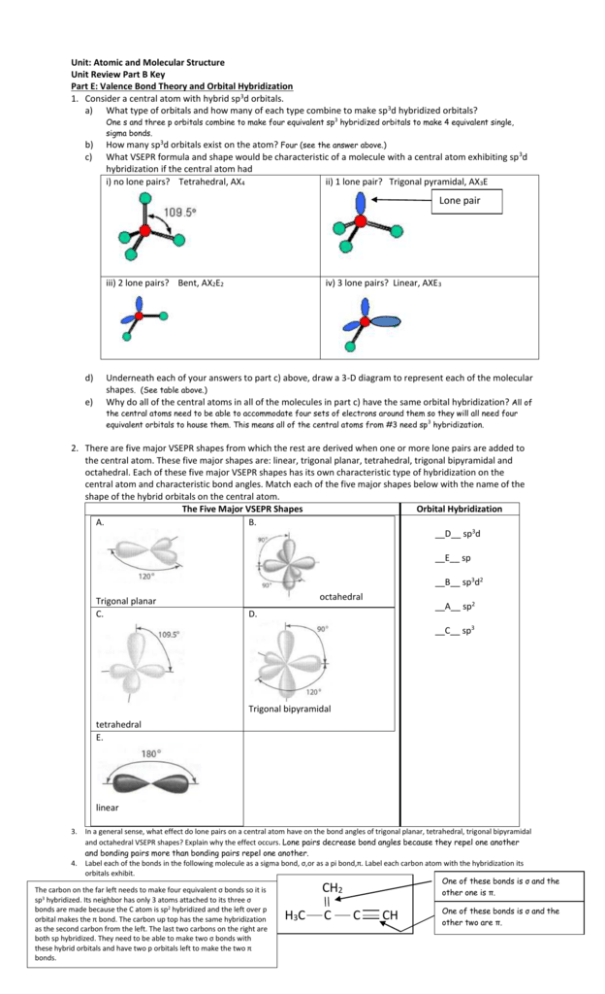
Unit: Atomic and Molecular Structure Unit Review Part B Key Part E: Valence Bond Theory and Orbital Hybridization 1. Consider a central atom with hybrid sp3d orbitals. a) What type of orbitals and how many of each type combine to make sp 3d hybridized orbitals? b) c) One s and three p orbitals combine to make four equivalent sp3 hybridized orbitals to make 4 equivalent single, sigma bonds. How many sp3d orbitals exist on the atom? Four (see the answer above.) What VSEPR formula and shape would be characteristic of a molecule with a central atom exhibiting sp 3d hybridization if the central atom had i) no lone pairs? Tetrahedral, AX4 ii) 1 lone pair? Trigonal pyramidal, AX3E Lone pair iii) 2 lone pairs? Bent, AX2E2 d) e) iv) 3 lone pairs? Linear, AXE3 Underneath each of your answers to part c) above, draw a 3-D diagram to represent each of the molecular shapes. (See table above.) Why do all of the central atoms in all of the molecules in part c) have the same orbital hybridization? All of the central atoms need to be able to accommodate four sets of electrons around them so they will all need four equivalent orbitals to house them. This means all of the central atoms from #3 need sp 3 hybridization. 2. There are five major VSEPR shapes from which the rest are derived when one or more lone pairs are added to the central atom. These five major shapes are: linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral. Each of these five major VSEPR shapes has its own characteristic type of hybridization on the central atom and characteristic bond angles. Match each of the five major shapes below with the name of the shape of the hybrid orbitals on the central atom. The Five Major VSEPR Shapes Orbital Hybridization A. B. __D__ sp3d __E__ sp __B__ sp3d2 Trigonal planar C. octahedral __A__ sp2 D. __C__ sp3 Trigonal bipyramidal tetrahedral E. linear 3. In a general sense, what effect do lone pairs on a central atom have on the bond angles of trigonal planar, tetrahedral, trigonal bipyramidal and octahedral VSEPR shapes? Explain why the effect occurs. Lone pairs decrease bond angles because they repel one another and bonding pairs more than bonding pairs repel one another. 4. Label each of the bonds in the following molecule as a sigma bond, σ,or as a pi bond,π. Label each carbon atom with the hybridization its orbitals exhibit. One of these bonds is σ and the The carbon on the far left needs to make four equivalent σ bonds so it is 2 other one is π. sp3 hybridized. Its neighbor has only 3 atoms attached to its three σ 2 5. Describe twoC atom differences between bonds are made because the is sp hybridized and sigma the left and over ppi bonds. One of these bonds is σ and the 3 orbital makes the π bond. The carbon up top has the same hybridization other two are π. as the second carbon from the left. The last two carbons on the right are both sp hybridized. They need to be able to make two σ bonds with these hybrid orbitals and have two p orbitals left to make the two π bonds. CH HC C C CH 5. Describe two differences between sigma and pi bonds. (See page 236.) Sigma bonds make single bonds. Pi bonds are involved in multiple bonds. Sigma bonds are end-to-end overlaps of orbitals. Pi bonds are side-to-side overlaps. 6. Define the terms “hybridization of orbitals” and “hybrid orbitals”. (See page 234.) When and why does hybridization occur? (Read page 233, “Hybrid Orbitals”.) Part F: Lewis Structures, VSEPR Theory, Molecular Polarity (There is another page to the answer key. The answers for #1 and #2. are there. 3. Textbook questions: #36, 37, 38 page 287 (Answers on page 816.) Part G: Intermolecular Forces 1. 2. 3. Explain the difference between intermolecular forces and intramolecular forces. Intramolecular forces are covalent bonds within a molecule. They are the bonds within the molecule that hold the atoms of the molecule together. Sometimes ionic bonds are considered intramolecular bonds, too, however ionic compounds are generally not molecules. Intermolecular forces are the forces of attraction between neighboring molecules. List the 3 different types of intermolecular forces and distinguish between them. The three types of intermolecular forces are London forces, dipole-dipole forces and a special type of dipole-dipole force called hydrogen bonding. All are covered in your grade 11 notes, in the grade 11 review notes given to you in this unit, on your second quiz and on pages 257-264 of the textbook. How are intermolecular forces linked to the properties of molecules? Again – this stuff can be found in the same places as the answers for #2. Summary: The stronger the intermolecular forces between neighboring molecules of the same type, the more energy required to separate the molecules from one another when changing state. So, generally, boiling points increase with the strength of intermolecular forces. Generally, increased intermolecular forces mean the molecules involved are more polar. The more polar the molecule, the higher its solubility tends to be in a polar solvent like water and the less soluble it tends to be in an non-polar solvent like a hydrocarbon (e.g. hexane(l)). 4. In Part F, question #1 you drew a 3-D diagram for SF6. Add bond dipoles and partial charges to a copy of the 3 – D diagram. State whether this molecule would be polar or non-polar and explain your reasoning. The bonds in this molecule are all polar covalent with the central atom, S, slightly positive compared to each F atom (all F atoms are slightly negative. All bond dipoles will move from the central S atom toward each F atom.) Because the molecule is symmetrical (all bonds involving S are to F atoms) and the central atom has no lone pairs, the bond dipoles will cancel. This molecule will be non-polar. 5. In Part F, question #1 you drew a 3-D diagram for BrF5. Add bond dipoles and partial charges to a copy of the 3 – D diagram. State whether this molecule would be polar or non-polar and explain your reasoning. The bonds in this molecule are all polar covalent with the central atom, Br, slightly positive compared to each F atom (all F atoms are slightly negative. All bond dipoles will move from the central Br atom toward each F atom.) In this case, the central atom has one lone pair so the molecule is no longer symmetrical and the bond dipoles do not cancel out. So – there is a net bond dipole and the molecule is considered a polar molecule. Lone pair 6a) Write in the bond dipoles and partial charges as necessary on the bonds in the molecules drawn below. State whether each molecule would be polar or non-polar. Identify all of the intermolecular forces that would exist between neighboring molecules of the same type in each case. Molar mass = 46 g/mol Molar mass = 46 g/mol Non- δ- symmetrical molecule with a net dipole so is a polar molecule. O H Polar molecule – same reasoning as for the first one. δ- C H Intermolecular forces between neighboring molecules of this type: London forces and dipole-dipole forces (because of the polar C=O bond) H C H N δ- H Also a polar molecule – same reasoning as above. O Intermolecular forces between neighboring molecules of this type: London forces, dipole-dipole forces and hydrogen bonding (because of the O-H bond) δ- O δ- Molar Mass = 45 g/mol Intermolecular forces between neighboring molecules of this type: 6b) Rank the three compounds from part a) from highest to lowest boiling point. Explain your answer. These molecules are all about the London forces, dipole- neighbors in samples of each molecule are about the diple and hydrogen same. (Review the factors affecting the strength of bonding (because of the London forces if this does not make sense to you.) N-H bond within the So, London force is not likely the deciding factor here molecule) in terms of predicting relative boiling points. Likely same size suggesting that the London forces between HCOOH will have the highest boiling point because its O-H bond is more electronegative than the N-H bond in the amide. They’ll likely be pretty close, though. The ketone will have the lowest of the three boiling points because although its does show dipole-dipole attraction between neighbors, there is no hydrogen bonding. 7. Textbook questions: page 266 #1-4; page 281 #18, 7. Textbook questions: page 266 #1-4 (See answers below.) ; page 281 #18, 20 (Answers in back of text.) ; page 289 #27 (an all-inclusive question) (See answers below.) Page 289 #27