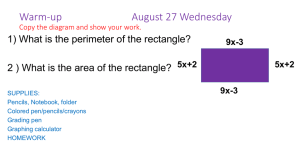
Bulk Polymerization of Styrene

Bulk Polymerization of Styrene
Bulk polymerization of styrene will be conducted with different initial initiator concentrations. The polymers will be analyzed for their MW and PDI by size exclusion chromatography (SEC) using a calibration curve. The MW dependence on conversion will be investigated. The effect of the initiator concentration on polymer MW will be examined.
Materials.
Azobisisobutyronitrile (AIBN) and toluene (HPLC) were purchased from Sigma-Aldrich and used as received. Styrene (Sigma-Aldrich) was purified by passing through a silica column to remove inhibitors. CDCl
3
was purchased from Cambridge Isotope and was used as received.
Methods.
Reaction procedure.
To a vial charged with freshly purified styrene (10 g) was AIBN (0.7g,
0.35g or 0.17g) added at room temperature. The mixtures are then heated at 80˚C in an oil bath.
Seven aliquots of the reaction mixture are removed at fixed time intervals (e.g., 0, 20, 40, 60,
80,100 min, 1 week) and analyzed by
1
H NMR spectroscopy for conversion and SEC for polymer MW and PDI.
Sample preparation for SEC analysis . An aliquot of the reaction mixture (3mg) is dissolved in
THF(1mL) and was filtered through a syringe membrane filter prior to injection into SEC column.
Sample preparation for
1
H NMR analysis . An aliquot of the reaction mixture (0.1-0.5 mg) is dissolved in CDCl
3
(1mL) and is transferred into a NMR tube for conversion analysis.
Lab report write-up .
Summarize and discuss your experimental results and describe the characterization techniques you used for this lab. The following questions should be addressed in the report.
(1) How does SEC characterization of polymer MW and PDI work? What are the advantage and limitation of this technique?
(2) How is a calibration curve constructed?
(3) What is the mechanism for the bulk polymerization of styrene?
(4) How did you obtain the conversion data?
(5) How does the polymer MW depend on conversions? Why?
(6) How does the polymer MW depend on the initiator concentration? why?
(7) Consider the major source of errors for the polymer MW and PDI obtained by SEC measurement using a calibration curve.
Answers for references only.
(1) Conventional SEC requires construction of calibration curve which correlates elution volume with MW. Every slice of SEC chromatograms can be analyzed by the calibration curve to get Mi. Intensity of the SEC chromatograms are typically an instrumental readout (e.g., DRI) that reflects the elute concentration (i.e., gi). So the M n
, M w
and PDI can be calculated from these (Mi, gi) values. The SEC analysis is a very time-efficient
1
approach to get MW and PDI information for high MW polymers. If the calibrants and polymers of interests are of identical type, the measured MWs are in the most part reliable, although PDI is typically over-estimated. However, if they are different, the MW values are relative MW as the calibrant and polymer of interests have different hydrodynamic volume. This approach requires the system to be stable (e.g., stable flow rate) and only size-exclusion being the operative separation mechanism.
(2) Calibration curve is typically built using a series of mono-disperse polymers with known
MWs covering a wide range (several KD to MD). Their peak elution time and log MW are fit with a polynomial function. This function is the calibration curve.
(3) We have been through this in class and exam. You should be able to write down equation depicting the initiation, propagation and termination steps.
(4) Conversion is defined as the mole fraction of monomers that are converted into polymers.
So it can be obtained from
1
H NMR by integrating peaks due to polymer repeating unit and unreacted monomers and compare their relative intensity.
(5) For all three runs (AIBN=0.7, 0.35, 0.17g), experimental results show that MW increase slightly with conversion at the beginning stage of the reaction (<50min). For the two runs with lesser AIBN initiators (AIBN= 0.35, 0.17g), very significant jump of MW is observed at the later stage of the reaction, where as the run with most AIBN
(AIBN=0.7g) exhibited consistently gradual increase.
4.8
4.6
4.4
4.2
Ingeo (AIBN=0.7g)
10
9
8
Mylar (AIBN=0.35g)
300
250
200
Kevlar (AIBN=0.17g)
4.0
3.8
7
150
100
3.6
3.4
3.2
20 30 40 50 60 70 80 90 100 conversion
6
5
10 20 30 40 50 60 70 80 90 conversion
50
0
0 10 20 30 40 conversion
50 60
DP
k p
[ M ]
2 ( fk t k d
[ I ])
1 / 2
The gradual MW increases at the low conversion is due to the change of [M]/[I] ratio over reaction time. Typically [I] decays more rapidly than [M], hence the slight increase of MW over time. The big jump of MW only happens for reactions that afford high MW polymers (i.e., smaller amounts of AIBN initiators). This is due to the gelation effect
(Trommsdorff effect). As high MW polymers are formed, viscosity of the reaction mixture increases. The termination becomes diffusion-controlled ( k d
is getting smaller).
Hence the MW increases significantly.
(6) The MW increases as the initiator AIBN amount decreases (see above equation).
(7) As mentioned in question 1, SEC system stability is important. As the MW relies on an accurate determination of elution volume, a constant flow rate is critical. Second, size
2
exclusion is the only operative mechanism for separation. (Imagine if there is affinity separation, elution volume will be greatly affected). If calibrant and polymer of interests are not identical type, their hydrodynamic volume will differ. Hence the MW obtained from the SEC method will be relative MW. Again, as we rely on converting elution volume into a MW value, the self-diffusion artificially broadens the MW distribution.
This cannot be avoided in SEC analysis using a calibration curve.
3