Eighth Grade Physical Science
advertisement
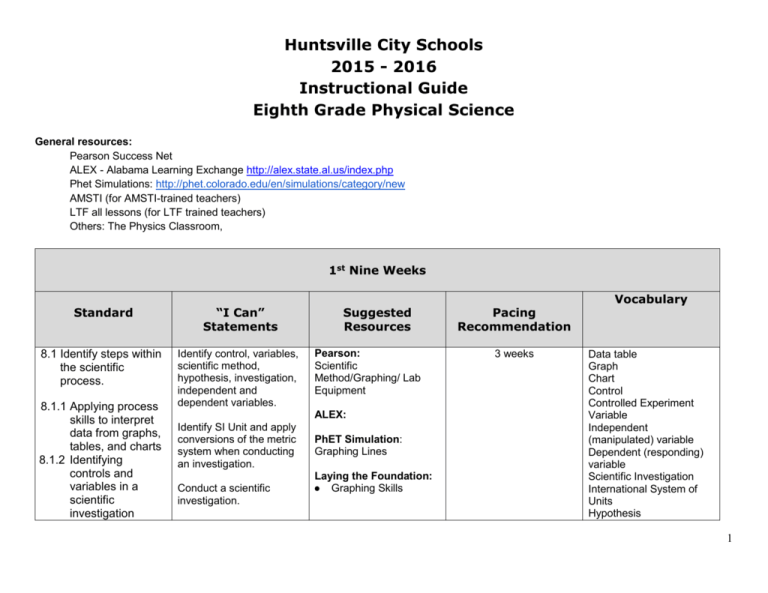
Huntsville City Schools 2015 - 2016 Instructional Guide Eighth Grade Physical Science General resources: Pearson Success Net ALEX - Alabama Learning Exchange http://alex.state.al.us/index.php Phet Simulations: http://phet.colorado.edu/en/simulations/category/new AMSTI (for AMSTI-trained teachers) LTF all lessons (for LTF trained teachers) Others: The Physics Classroom, 1st Nine Weeks Standard “I Can” Statements 8.1 Identify steps within the scientific process. Identify control, variables, scientific method, hypothesis, investigation, independent and dependent variables. 8.1.1 Applying process skills to interpret data from graphs, tables, and charts 8.1.2 Identifying controls and variables in a scientific investigation Suggested Resources Pearson: Scientific Method/Graphing/ Lab Equipment ALEX: Identify SI Unit and apply conversions of the metric system when conducting an investigation. Conduct a scientific investigation. PhET Simulation: Graphing Lines Laying the Foundation: ● Graphing Skills Pacing Recommendation 3 weeks Vocabulary Data table Graph Chart Control Controlled Experiment Variable Independent (manipulated) variable Dependent (responding) variable Scientific Investigation International System of Units Hypothesis 1 8.1.4 Identifying examples of hypotheses 8.1.5 Identifying appropriate laboratory glassware, balances, time measuring equipment, and optical instruments used to conduct an investigation 8.8 Identify Newton's three laws of motion. 8.8.1 Defining terminology such as action and reaction forces, inertia, acceleration, momentum, and friction 8.8.2 Interpreting distance-time graphs Apply process skills to interpret data from graphs, tables, and charts ● ● Generate tables and graphs based on information gathered during an investigation. Identify appropriate lab equipment including glassware, balances, and time measuring equipment use and conducting an investigation. Determine when an object is in motion relative to a reference point. Measure distance using appropriate SI units. Describe and compare speed, velocity, and acceleration. Graph and interpret distance-time graphs and calculate slope. Identify action, reaction forces, inertia, acceleration, momentum, friction. List the three Laws of Motion. Scientific Method (Come Fly with Me/Penny Lab) Whirligig Lollapalooza AMSTI: Experimenting with Forces and Motion Other: The Gummy Bear Lab http://sciecnespot.net/Med ia/mmaniabearlab.pdf Pearson: Chapter 7: Motion, Lessons 1-3 Chapter 8 Forces, Lessons1-4 ALEX: The Need for Speed PhET Simulation(s): Forces and Motion (Basics) The Moving Man Position vs Time Graphs Velocity vs Time Graphs Laying the Foundation: The Force to be Reckoned With Gravity Force Lab 4 weeks Newton’s Three Laws of Motion Action Reaction Inertia Acceleration Momentum Friction Motion Reference Point Distance Speed Average Speed Instantaneous Speed Velocity Slope Acceleration Gravity Weight Mass Law of Conservation of Momentum Net Force 2 Apply measurement skills to determine the motion of an object as it accelerates. Calculate momentum. Describe how forces affect motion. Velocity vs. Time Graphs -Describing Motion Speed - Measuring Constant Speed Ramped Up - Setting the Wheels in Motion AMSTI: Experimenting with Forces and Motion Identify the forces acting upon an object. Define gravity, weight, and mass. Benchmark Review and Assessment 1 week 3 2nd Nine Weeks Standard 8.9. Describe how mechanical advantage of simple machines reduce the amount of force needed for work. “I Can” Statements Resources Identify and calculate work and power. Pearson: Ch 9 Work and Machines Identify simple and compound machines. ALEX: Inventions using Simple Machines Projects How to make something … simple Incline plane and the crashing marble Identify the input and output force of a simple machine. Laying the Foundation: Levers are Us AMSTI: Working with motors and simple machines Pacing Recommendation 3 Weeks Vocabulary Energy Work Joule Power Watt Force Mechanical Advantage Simple Machines Incline Planes Wedge Screw Lever Fulcrum Pulley Wheel and axle Input force Output force Compound machines Efficiency PhET Simulations: The Ramp Others: Ed Heads Simple Machines and Compound Machines http://www.edheads.org/a ctivities/simple-machines/ Rube-Goldberg Machines Project 4 8.10 Differentiate between potential and kinetic energy. Compare potential and kinetic energy. Describe how energy and work are related. Pearson: Chapter 10 Energy Lesson 1 1 weeks Energy Kinetic Energy Potential Energy Gravitational Potential Energy Elastic Potential Energy Mechanical Energy 1 week Energy transformation Law of Conservation of Energy Mechanical Energy Nuclear Energy Thermal Energy Electrical Energy Radiant Energy Chemical Energy 3 weeks Mechanical Waves Electromagnetic Waves Longitudinal Waves ALEX PhET Simulations: Energy Skate Park Energy Forms and Changes Laying the Foundation: Roller Coaster Fun AMSTI: Working With Motors and Simple Machines 8.11 Explain the law of conservation of energy and its relationship to energy transformation, including chemical to electrical, chemical to heat, electrical to light, electrical to mechanical, and electrical to sound Describe how energy is conserved in a transformation. Pearson: Chapter 10 Energy Lesson 2-3 Give examples of energy transformations for the different forms of energy. ALEX: 8.12 Classify waves as mechanical or electromagnetic. Identify waves as electromagnetic or mechanical. Laying the Foundation: Cool Chemical Reactions Others: http://www.science4us.co m/elementary-physicalscience/energy/energytransformations/ Pearson: Chapters 12 Characteristics of Waves 5 Examples: - mechanicalearthquake waves; - electromagneticultraviolet light waves, visible light waves 8.12.1 Describing how earthquake waves, sound waves, water waves, and electromagnetic waves can be destructive or beneficial due to the transfer of energy 8.12.2 Describing longitudinal and transverse waves 8.12.3 Describing how waves travel through different media 8.12.4 Relating wavelength, frequency, and amplitude to energy 8.12.5 Describing the electromagnetic Identify wavelength, frequency, and amplitude of a wave. Describe the effect of different types of waves (electromagnetic, longitudinal, transverse, sound, earthquake waves) in the way they transfer energy. Ch 13 Sound Ch 14 Electromagnetic Waves ALEX Transverse Waves http://alex.state.al.us/lesso n_view.php?id=24112 Transverse waves Standing Waves Wavelength Frequency Amplitude Electromagnetic Spectrum Sound Waves Seismic Waves Phet Wave on a string Describe how waves travel through different media. Laying the Foundation: Catch the Wave: Standing Wave Patterns Describe the electromagnetic spectrum in terms of frequencies. AMSTI: Working with Motors and Simple Machines 6 spectrum in terms of frequencies Example: electromagnetic spectrum in increasing frequenciesmicrowaves, infrared light, visible light, ultraviolet light, X rays Benchmark Review and Assessment 1 week 7 3rd Nine Weeks Standard 8.7 Describe states of matter based on kinetic energy of particles in matter. “I Can” Statements Compare and contrast the states of matter based on the levels of kinetic energy. Interpret an illustration of states of matter. Resources Pearson: Ch. 1 Intro. To Matter Chapter 2 Solids, Liquids and Gases Lesson 1-3 ALEX PhET Simulations: States of Matter Collisions Laying the Foundation States of Matter Evaporation and Condensation AMSTI: Exploring the Properties of Matter Pacing Recommendation 2 weeks Vocabulary Matter Physical Property Chemical Property Physical Change Chemical Change Solid Liquid Gas Surface Tension Viscosity Pressure Melting/Freezing Point Vaporization Condensation Vaporization Sublimation Boiling Point Other: Volume and Measurement Review http://www.commoncoresh eets.com 8 8.1.2 Measuring dimension, volume, and mass using Système International d'Unités (SI units) Describe and measure SI units used to measure mass, volume and density. Pearson: Calculate Density Laying the Foundation: Chromatography of Drink Mixes (Separating Dyes) 1 week Volume Mass Density 1 week Buoyant force Fluid Pressure 2 weeks Democritus Dalton Ch 1 Introduction to Matter Lesson 3 AMSTI: Exploring the Properties of Matter PhEt: Density 8.9.1 Describing the effect of force on pressure in fluids Explain the relationship between fluid and pressure. Explain the relationship between fluid pressure and buoyant force. Other: Volume and Measurement Review www.commoncoresheets.c om Pearson: Ch 8 Sinking and Floating Lesson 3 Laying the Foundation: Building a paper Airplane using Bernoulli’s Principle AMSTI: Exploring the Properties of Matter PhEt: Density 8.2. Describe the structure of atoms, Draw and label the parts of the atom. Other: Float or Sink from Planet diary 1.3 Pearson: 9 including the location of protons, neutrons, and electrons. 8.2.1 Identifying the charge of each subatomic particle 8.2.2 Identifying Democritus and Dalton as contributors to the atomic theory Identify all the subatomic particles of the atom. Compare and contrast Democritus and Dalton’s contributions to the atomic theory. Understand the information provided on the periodic table. Ch 3 Elements and the Periodic Table Atoms Protons Neutrons Electrons Electron Cloud Subatomic Particles Atomic Theory Mass Number Atomic Number/Weight Periodic Table ALEX Get in the Mix Laying the Foundation: Making Sense of that Chart on the Wall AMSTI: Exploring the Properties of Matter Others: http://www.iknowthat.com/ mhscience/Atoms/Fixed.ht m 8.3 Determine the number of protons, neutrons, and electrons, and the mass of an element using the periodic table. Determine the number of protons, neutrons, and electrons in a given atom. Pearson: Ch. 3 Elements and Periodic Table Locate and identify metals, nonmetals, metalloids and noble gases on the periodic table. ALEX 8.3.1 Locating metals, nonmetals, metalloids, and noble gases on the periodic table 8.3.2 Using data about the number of electrons in the outer shell of an Define valence electrons and identify the valence electrons for each group on the periodic table. Determine the reactivity of an atom using the periodic table. Laying the Foundation: * Why do They Call It A Periodic Table? AMSTI: Exploring the Properties of Matter PhET Simulation: Build an Atom http://phet.colorado.edu/ 2 weeks Dimitri Mendeleev Atomic Mass Chemical Symbol Group Period Periodic Table Valence Electrons Alkali Earth Metals Alkali Metals Metals Nonmetals Metalloids Noble Gases Outer Shells Reactivity Malleable Ductile Corrosion Other: 10 atom to determine its reactivity Benchmark Review and Assessment The Science Spot: Chemistry – Matter, Atoms, & More http://www.sciencespot.net /Pages/kdzchem.html 1 week 11 4th Nine Weeks Standard 8.5 Differentiate between ionic and covalent bonds. 8.5.1 Illustrating the transfer or sharing of electrons using electron dot diagrams “I Can” Statements Resources Pacing Recommendation Describe how electrons are transferred between atoms to create new compounds. Pearson: Ch. 4 Atoms and Bonding 2.5 weeks Differentiate between ionic and covalent bonds, and illustrate the sharing of electrons using electron dot diagrams. PhET Build a Molecule Draw the valence electrons of an atom using the Lewis Dot model. Use electron dots to show how atoms bond to each other and to different atoms. ALEX Laying the Foundation: Chemical Nomenclature AMSTI: Mixtures, Compounds and Elements Vocabulary Chemical Bonds Chemical Formula Ion Ionic Bonds Covalent Bonds Metallic Bonds Valence Electrons Electron Transfer Electron Dot Diagram Polar Bond Non Polar Bond Single Bond Double Bond Triple Bond Subscript Cation Anion Other: Bonding Basics http://www.sciencespot.net /Media/bondingbasics08_w kst.pdf Candy Compounds http://www.sciencespot.net /Media/candycompounds.p df 12 8.4 State the law of conservation of matter. Identify the components of a chemical reaction. 8.4.1 Balancing chemical equations by adjusting coefficients State the law of conservation of matter. Balance equations and identify the type of reaction in the equation. Pearson: Chapter 5 Chemical Reactions ALEX PhET Simulations: Reactants, Products, and Leftovers Balancing Chemical Equations 2.5 weeks Reactant Product Exothermic Reaction Endothermic Reaction Chemical Equation Law of Conservation of mass Coefficient Synthesis Reaction Decomposition Reaction Replacement Reaction Activation Energy Laying the Foundation: Cool Chemical Reaction AMSTI: Mixtures, Compounds and Elements Other: New Bubble Gum Lab http://www.teacherweb.co m/FL/StonemanDouglasH S/.../-NEW-bubble-gumlab.pdf Science Spot Chemical Reactions Balancing Act 13 Balancing Equations Online http://www.sciencespot.net /Pages/classchem.html 8.7.1 Explaining effects of temperature, concentration, surface area, and catalysts on the rate of chemical reactions Define rate of a reaction. Describe the effects that catalysts, inhibitors, surface area, and concentration have on the rate of chemical reactions. Pearson: Chapter 5 Chemical Reactions Lesson 3 ALEX PhET Simulations: Reaction Rates 1 week Reaction Rate Activation Energy Concentration Surface Area Catalyst Endothermic Reaction Exothermic Reaction Inhibitor Enzyme Laying the Foundation: Where’s The Heat?: Investigating Exothermic and Endothermic Processes AMSTI: Mixtures, Compounds and Elements Other: New Bubble Gum Lab http://www.teacherweb.co m/FL/StonemanDouglasH S/.../-NEW-bubble-gumlab.pdf 14 8.6 Define solution in terms of solute and solvent. 8.6.1 Defining diffusion and osmosis 8.6.2 Defining isotonic, hypertonic, and hypotonic solutions 8.6.3 Describing acids and bases based on their hydrogen ion concentration Define solution, solute and solvent. Pearson: Chapter 6 Acids, Bases and Solutions Define and compare diffusion and osmosis. ALEX Describe properties of acids and bases including pH, taste, feel, and reactivity. Phet Sugar and Salt Solutions (also OK with ionic/covalent bonding) Use pH to compare acidity and strength of solutions. Laying the Foundation: pH – Developing the Concept of pH Sugar and Salt Solutions (also OK with ionic/covalent bonding) 2 weeks Other: Osmosis and Diffusion in an Egg http://www.biologyjunction. com/osmosis_diffusion_in_ egg_lab.htm Solute Solvent Solution Colloid Suspension Dilute Solution Concentrated Solution Saturated Solution Solubility Corrosive Indicator Diffusion Osmosis Isotonic Hypotonic Hypertonic Acid Base Hydrogen Ion Hydroxide Ion pH Scale Neutralization Salt GEMS Alíen Juice Bar http://scienceview.berkeley .edu/showcase/flash/juiceb ar.html Cabbage Indication pH Lab www.middleschoolscience. com/cabbage.htm Benchmark 1 week 15 Various Activities for finish year 1 week 16