Chemistry 30
advertisement

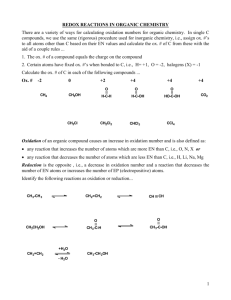
Chemistry 30 Electrochemistry Notes A. Redox Reactions 30-B1.1k define oxidation and reduction operationally and theoretically 30-B1.2k define oxidizing agent, reducing agent, oxidation number, half-reaction, disproportionation 30-B1.7k – write and balance equations for redox reactions in acidic and neutral solutions by…developing simple half-reaction equations from information provided about redox changes electrochemistry is the branch of chemistry that studies _____________________ __________________________________________________________________ _________________ is a _____________________________________________ eg) __________________ is a ____________________________________________ eg) oxidation and reduction reactions occur together, hence the term ______________ the reduction and oxidation reactions are called the ________________________ “adding” the half reactions together will give you the ______________________ ____________________________ that takes place during the redox reaction the e lost in the oxidation half reaction _______________________ the e gained in the reduction half reaction you may have to ________________________________________________ of the half reactions to balance the e _____________________________________ (ions not changing) are _________ included! the substance that is ________________________ is called the _____________________________________________…it causes the oxidation by taking e the substance that is _________________________ is called the _____________________________________________…it causes the reduction by giving up e Chemistry 30 Electrochemistry 1 Example 1 Given the following reaction, write the half reactions and the net ionic equation. Na(s) + LiCl(aq) Li(s) + NaCl(aq) Example 2 Given the following reaction, write the half reactions and the net ionic equation. 3 Zn(s) + 2 Au(NO3)3(aq) 2 Au(s) + 3 Zn(NO3)2(aq) Example 3 Given the following reaction, write the half reactions and the net ionic equation. Br2(l) + 2 NaI(aq) I2(s) + 2 NaBr(aq) Your Assignment: pg 1 “Net Ionic Equations” #1-5 Chemistry 30 Electrochemistry 2 B. Spontaneous Redox Reactions 30-B1.6k predict the spontaneity of a redox reaction, based on standard reduction potentials, and compare their predictions to experimental results chemical reactions which occur on their own, without the input of ____________________________________________, are called ________________________________ not all reactions are spontaneous in the table of redox half reactions (see pg 9 in Data Booklet), the _____________________________________________ is at the top left and the ____________________________________________ is at the bottom right the ________________________________________ rule states that a spontaneous reaction occurs if the ____________________ agent is above the _______________ agent in the table of redox half reactions Try These: For each of the following combinations of substances, state whether the reaction would be spontaneous or non-spontaneous: 1. Cr3+(aq) with Ag(s) 2. I2(s) with K(s) 3. H2O2(l) with Au3+(aq) 4. Sn2+(aq) with Cu(s) 5. Fe2+(aq) with H2O(l) C. Predicting Redox Reactions 30-B1.6k predict the spontaneity of a redox reaction, based on standard reduction potentials, and compare their predictions to experimental results 30-B1.7k – write and balance equations for redox reactions in acidic and neutral solutions by…using halfreaction equations from a standard reduction potential table 30-B1.2s use a standard reduction potential table as a tool when considering the spontaneity of redox reactions and their products 30-B2.7k – predict the spontaneity or nonspontaneity of redox reactions, based on the relative positions of half-reaction equations on a standard reduction potential table we will be predicting the strongest or most dominating reaction that occurs when substances are mixed (other reactions do take place because of atomic collisions!) Chemistry 30 Electrochemistry 3 Steps: 1. List all species present as reactants 1. dissociate _____________________________________ and _______________ 2. do not dissociate ____________________________________ 3. include ____________ ions if it is _________________ 4. always include _________________ 2. Identify each as ________ or _________ (***some can be both so memorize them…_______________________________________) 3. Identify the _______ and __________ using the table (SOA is closest to top, SRA is closest to bottom). 4. Write out the ______________________ for the SOA and SRA. 5. Determine the ________________________________________. 6. Determine ________________________ (SOA must be higher than SRA to be spontaneous) Example 1 Predict the most likely redox reaction when chromium is placed into aqueous zinc sulphate. Example 2 Predict the most likely redox reaction when silver is placed into aqueous cadmium nitrate. Chemistry 30 Electrochemistry 4 Example 3 Predict the most likely redox reaction when potassium permanganate is slowly poured into an acidic iron (II) sulphate solution. Your Assignment: pgs 1-2 “Predicting Redox Reactions” #1-15 D. Generating Redox Tables you can be given data for certain ions and elements then be asked to generate a redox table like the one on pg 9 of you Data Booklet (a smaller version!) you may have to generate a table from real or fictional elements and ions the tables that we use are all written as _______________________ half reactions Example 1 Generate a redox table given the following data: indicates no reaction indicates a reaction Cu2+(aq) Zn2+(aq) Pb2+(aq) Ag+(aq) Cu(s) Zn(s) Pb(s) Ag(s) Redox Table Put the oxidizing agents in order from strongest to weakest. Put the reducing agents in order from strongest to weakest. Chemistry 30 Electrochemistry 5 Example 2: Generate a redox table given the following data: Cu(s) + Ag+(aq) Cu2+(aq) + Ag(s) Zn2+(aq) + Ag(s) no reaction Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s) Hg(l) + Ag+(aq) no reaction Redox Table Put the oxidizing agents in order from weakest to strongest. Put the reducing agents in order from weakest to strongest. Example 3 Generate a redox table given the following data: 2X(aq) + Y2(g) spontaneous reaction 2Z(aq) + Y2(g) no reaction 2Z(aq) + W2(g) spontaneous reaction Redox Table Put the oxidizing agents in order from strongest to weakest. Put the reducing agents in order from strongest to weakest. Chemistry 30 Electrochemistry 6 Your Assignment: pgs 2-3 “Generating Redox Tables” #1 - 5 E. Oxidation Numbers (States) 30-B1.2k define oxidizing agent, reducing agent, oxidation number, half-reaction, disproportionation 30-B1.7k – write and balance equations for redox reactions in acidic and neutral solutions by… an _________________________ is the charge an atom ___________ to have when found in a __________________________________ or charged _______________________________________ can be used when you have a ______________________________ where there are no ________________________ to determine if oxidation or reduction is occurring how do you use a change in the number? 1. if the number __________________ then __________________ has occurred 2. if the number __________________ then __________________ has occurred Rules for Assigning Oxidation Numbers: 1. In a pure element, the oxidation number is ____________. 2. In simple ions, the oxidation number is equal to the ________________. 3. In most compounds containing hydrogen, the oxidation number for hydrogen is ________. (Exception is the metal hydrides eg) LiH where the oxidation number of hydrogen is _________) 4. In most compounds containing oxygen, the oxidation number for oxygen is __________. (Exception is the peroxides eg) H2O2, Na2O2 where the oxidation number of oxygen is __________) 5. The sum of oxidation numbers of all atoms in a substance must equal the ___________________ of the substance. (______________ for compounds and __________________ of the polyatomic ion) eg) sum of MgO = _______ sum of PO43 = ________ Example What is the oxidation number (state) for the element identified in each of the following substances? a) N in N2O Chemistry 30 Electrochemistry 7 b) N in NO3 c) C in C2H5OH d) C in C6H12O6 30-B1.3k differentiate between redox reactions and other reactions, using half-reactions and/or oxidation numbers 30-B1.4k identify electron transfer, oxidizing agents and reducing agents in redox reactions that occur in everyday life, in both living systems (eg. cellular respiration, photosynthesis) and nonliving systems; i.e., corrosion figuring out oxidation numbers can help to identify whether a reaction is a ________________________________________________ or not for it to be a redox reaction, there has to be ___________ an ________________ in oxidation number and a ________________ in oxidation number seen in the reaction eg) Ag(s) + NaNO3(aq) Na(s) + AgNO3(aq) PbSO4(aq) + 2 KI(aq) PbI2(s) + K2SO4(aq) electron transfer occurs in ___________________________________ eg) also occurs in _____________________________________________ eg) Chemistry 30 Electrochemistry 8 F. Disproportionation 30-B1.2k define oxidizing agent, reducing agent, oxidation number, half-reaction, disproportionation disproportionation occurs when one element _______________________ __________________________________________________________ eg) 2 H2O2(aq) 2 H2O(l) + O2(g) Cl2(g) + 2 OH(aq) ClO(aq) + Cl(aq) + H2O(l) Your Assignment: pg 4 “Oxidation Numbers” #1-5 G. Balancing Redox Reactions 30-B1.7k – write and balance equations for redox reactions in acidic and neutral solutions by…assigning oxidation numbers, where appropriate, to the species undergoing chemical change sometimes most reactants and products are known but the complete reaction is not given…called a ________________________ reaction 1. Half Reaction Method 1. Assign __________________________________________. 2. Balance the _________________ that changes in oxidation number. 3. Add _______ to balance the change in _________________ oxidation number (______________________________________________). 4. Balance O using _________. 5. Balance H using _________. 6. Check that the half-reaction is balanced with respect to ___________. Example 1 Balance the following half reaction: CrO42(aq) Chemistry 30 Electrochemistry 9 CrO2(aq) Example 2 Balance the following half reaction: HClO2(aq) Cl2(g) Your Assignment: pg 5 “Balancing Redox Half Reactions” #1-10 now we are going to combine coming up with our own half reactions with figuring out the net redox reaction Steps 1. Assign _________________________________________. 2. Separate the partial net equation into two _______________________ (omit any _______ or _______). 3. Balance each half-reaction. 4. _________________________________________ of the equations so e lost = e gained 5. Add the equations to produce a balanced _______________________. 6. __________________. Check to see if all elements and charges are balanced. Example 1 Balance the following reaction using the half reaction method:: ___H+(aq) + ___CrO42(aq) + ___SO32-(aq) ___CrO2(aq) + ___SO42(aq) + ___H2O(l) Your Assignment: pg 5 “Half Reaction Method” #1-6 Chemistry 30 Electrochemistry 10 2. Oxidation Number Method we will use the unbalanced reaction and the change in oxidation numbers to work out the balancing Steps: 1. Assign oxidation numbers. 2. Balance the substances that change in oxidation number. 3. Use a _________ to join the reducing agent with its corresponding product (ignore the H+(aq) and H2O(l)) and a ________ to join the oxidizing agent with its corresponding product. 4. On each line, write the ___________ in oxidation number # of atoms. 5. ___________ the RA and/or OA to balance the change in oxidation number. 6. ____________________ the H2O(l) and the H+(aq). Example 1 Balance the following reaction using the oxidation number method. __H+(aq) + __MnO4(aq) + __SO32(aq) __MnO2(s) + __SO42(aq) + __ H2O(l) Example 2 Balance the following reaction using the oxidation number method. __ H2O(l) + __N2O4(g) + __ Br(aq) __NO2 (aq) + __ BrO3(aq) + __ H+(aq) Your Assignment: pg 5 “Oxidation Number Method” #1-6 H. Redox Stoichiometry 30-B1.8k perform calculations to determine quantities of substances involved in redox titrations 30-B1.4s select and use appropriate numeric, symbolic, graphical and linguistic modes of representation to communicate equations for redox reactions and answers to problems related to redox titrations Chemistry 30 Electrochemistry 11 1. Calculations stoichiometry can be used to predict or analyze a quantity of a chemical involved in a chemical reaction in the past we have used balanced chemical equations to do stoich calculations we can now apply these same calculations to balanced redox equations Example 1 What is the mass of zinc is produced when 100 g of chromium is placed into aqueous zinc sulphate. Example 2 What volume of 1.50 mol/L potassium permanganate is needed to react with 500 mL of 2.25 mol/L acidic iron (II) sulphate solution? Your Assignment: pgs 6 “Redox Stoichiometry” #1-6 Chemistry 30 Electrochemistry 12 2. Titrations a titration is a lab process used to determine the _________________ of a substance needed to react completely with another substance this volume can then be used to calculate an unknown _______________________ using stoichiometry one reagent (_________________ - ________) is slowly added to another (____________________ - __________) until an abrupt change (____________________) occurs, usually in colour in redox titrations, two common oxidizing agents are used because of their ____________________and _____________________: i. ii. as long as the sample (RA) in the flask is reacting with the ________________________________________________ the sample will be ______________________ when the reaction is complete, any unreacted permanganate ions will turn the sample ____________________ (pink) (with dichromate, sample goes from orange to green) the volume of titrant (OA) needed to reach the endpoint is called the ________________________________ the __________________________________of the titrant must be accurately known the concentration of the permanganate solution must be calculated against a ______________________________________ (a solution of known concentration) before it can be used in a titration itself this is done just prior to the titration Chemistry 30 Electrochemistry 13 Example Find the concentration of (standardize) the KMnO4(aq) solution by titrating 10.00 mL of 0.500 mol/L acidified tin (II) chloride with the KMnO4(aq). Evidence Trial 1 2 3 4 Final Volume (mL) 18.40 35.30 17.30 34.10 Initial Volume 1.00 18.40 0.60 17.30 pink light pink light pink light pink (mL) Volume of _________ (mL) Endpoint Colour endpoint average is calculated by using 3 volumes within 0.20 mL Endpoint average = Your assignment: pg 6 “Redox Stoichiometry” #7-9 Chemistry 30 Electrochemistry 14 I. Electrochemical Cells 1. Voltaic Cells 30-B2.1k define anode, cathode, anion, cation, salt bridge/porous cup, electrolyte, external circuit, power supply, voltaic cell and electrolytic cell _______________ are devices that convert ____________ energy into _____________ energy in redox reactions, e are transferred from the ___________________ to the ___________________________ the transfer of e can occur through a __________________________ separating the two substances in containers called ________________ a ___________________________________ is an arrangement where _______________________________ are joined so that the ________ and ________________ can move between them _________________________ are made of good conducting materials so e can flow…can be the _________________of the solution or inert such as _______________________ the ________________________________ is a solution that contains ions and will transmit ions the electrode where _______________ occurs is called the __________________ if the anode is a metal, it _________________ mass as the cell operates the anode is labelled as _________________________ since it is the electrode where electrons originate the __________________ move to the _______________ since this electrode ________ electrons (leaving a net ___________________ charge in the electrode) the electrode where _______________ occurs is called the __________________ if the cathode is a metal, it _________________ mass as the cell operates the cathode is labelled as _________________________ since the anode is labelled negative the __________________ move to the _______________ since this electrode _______________ electrons (leaving a net ______________ charge in the electrode) Chemistry 30 Electrochemistry 15 electrons flow from the ______________________________ to the ____________________________ through a connecting wire ions must be able to ____________ to their attracting electrode (either through the _______________________ or a _________________) otherwise a buildup of charge will occur opposing the movement of e the flow of ions through the solution and e through the wire maintains overall __________________________________________ 2. Standard Reduction Potentials (E) 30-B2.5k explain that the values of standard reduction potential are all relative to 0 volts, as set for the hydrogen electrode at standard conditions ________________________________ are the ability of a half cell to ______________________________________ these potentials are measured using a __________________________ each half reaction listed in the Data Booklet has an E value measured in ___________________assigned to it all values in the table are arbitrarily assigned based on a standard the _________________________________________ half reaction has been set as the standard and has an E value of ______________ 3. Predicting Voltage of a Voltaic Cell 30-B2.3k predict and write the half-reaction equation that occurs at each electrode in an electrochemical cell 30-B2.6k – calculate the standard cell potential for electrochemical cells the standard cell potential ____________ is determined by _________ the ______________________ for the two half reactions the ____________ on the E value for the ____________________ half reaction must be ____________________________ if you multiply an equation to balance e, you ___________________ multiply the E value (voltage is independent of number of e transferred) a ____________________ E net is a _____________________ reaction a ____________________ E net is a _____________________ reaction Chemistry 30 Electrochemistry 16 Example Calculate the Enet for the reaction of Zn(s) with CuSO4(aq). 4. Shorthand Notation or line ___________ separates_______________ double line __________ represents the ____________________ or _____________________ and separates the two _________________ comma _______separates ___________________________________ ___________________________________ 5. Drawing Cells 30-B2.3k predict and write the half-reaction equation that occurs at each electrode in an electrochemical cell 30-B2.6k – calculate the standard cell potential for electrochemical cells 30-B2.7k – predict the spontaneity or nonspontaneity of redox reactions, based on standard cell potential when drawing a cell from the shorthand notation, you have to be able to label the cathode, anode, positive terminal, negative terminal, electrolytes, direction of e flow, and directions of cation and anion flow you also have to show and label the reduction half reaction, oxidation half reaction and net reaction including E values, E net and spontaneity Chemistry 30 Electrochemistry 17 Example Draw and fully label the following electrochemical cell: 3+ 2+ Al(s) /Al (aq)// Ni (aq) /Ni(s) Your Assignment: pg 7 “Electrochemical Cells” #1-8 Electrochemical Cells Lab J. Commercial Cells 30-B2.1sts explain that scientific knowledge may lead to the development of new technologies, and new technologies may lead to or facilitate scientific discovery 30-B2.3sts explain that science and technology have influenced, and been influenced by, historical development and societal needs __________________________are made by connecting two or more voltaic cells in ____________________________________________ the _______________________ of the battery is the ____________ of the voltages of the _________________________________________ there are many types of batteries: a) Dry Cell common __________________________ batteries of clocks, remote controls, noisy kids toys etc. Chemistry 30 Electrochemistry 18 Cathode (Red): 2 MnO2(s) + H2O(l) + 2e Mn2O3(aq) + 2 OH (aq) E= +0.79 V Anode (Oxid): E = 2+ Net: 2 MnO2(s) + H2O(l) + Zn(s) Mn2O3(aq) + 2 OH (aq) + Zn (aq) Enet = +1.55 V the __________ produced causes irreversible side reactions to occur making recharging impossible b) Nickel-Cadmium one type of ___________________________ battery Cathode (Red): 2 NiO(OH)(s) + 2 H2O(l) + 2e 2 Ni(OH)2(s) + 2 OH (aq) E= +0.49 V Anode (Oxid): Net: Cd(s) + 2 OH (aq) 2 Cd(OH)2(s) + 2e 2 NiO(OH)(s) + 2 H2O(l) + Cd(s) 2 Ni(OH)2(s) + Cd(OH)2(s) E = +0.76 V Enet = +1.25 V c) Lead Storage Battery __________________________________ where ___________ serves as the anode, and ____________________________________ serves as the cathode both electrodes dip into an electrolyte solution of ________________ ______________________ _______________________________ are connected in series Cathode (Red): PbO2(s) + HSO4 (aq) + 3 H+(aq) + 2e PbSO4(s) + 2 H2O(l) E= +1.68 V Anode (Oxid): Net: Pb(s) + HSO4 (aq) PbSO4(s) + H+(aq) + 2e + Pb(s) + PbO2(s) + 2 H (aq) + 2 HSO4 (aq) 2 PbSO4(s) + 2 H2O(l) E = +0.36 V Enet = +2.04 V d) Fuel Cells cells where reactants are _____________________________________________ Chemistry 30 Electrochemistry 19 the energy from this reaction can be used to ______________________________ one type is the _____________________________________________________ _________________________ is pumped in at the _______________ while ______________________ is pumped in at the _____________ (which both have a lot of surface area) pressure is used to push the H2 through a platinum catalyst which splits the H2 into 2H+ and 2e the 2e move through an external circuit towards the cathode generating electrical energy the O2 is also pushed through the platinum catalyst forming two oxygen atoms the H+ ions and oxygen atoms combine to form water Cathode (Red): O2(g) + 4 H+(aq) + 4e- 2 H2O(l) Anode (Oxid): 2 H2(g) 4 H+(aq) + 4e E= E = V V Net: need a source of hydrogen…reformers are used to convert CH4 or CH3OH into ___________ and ______________ unfortunately, __________ is a _______________________________________ about 24-32% efficient where gas-powered car is about 20% efficient K. Electrolytic Cells 1. The Basics 30-B2.1k define anode, cathode, anion, cation, salt bridge/porous cup, electrolyte, external circuit, power supply, voltaic cell and electrolytic cell in an electrolytic cell, ____________________ energy is used to force a ____________________________________ chemical reaction to occur (opposite of a voltaic cell) these reactions have a __________________________________Enet Chemistry 30 Electrochemistry 20 commonly used to _________________________________________________ (eg. gold, silver, bronze, chromium etc), _________________________________ and split compounds into ____________________________ (eg. H2, O2, Cl2 etc) the electrolytic cell is hooked up to a ____________________________________ _________________________________(instead of load or external circuit) so the flow of e is __________________________________ the __________________________ of the electrolytic cell is connected to the ____________________ of the battery and therefore is _____________________ the __________________________ of the electrolytic cell is connected to the ________________________ of the battery and therefore is ________________ 30-B2.2k identify the similarities and differences between the operation of a voltaic cell and that of an electrolytic cell Voltaic Cells Voltaic vs. Electrolytic Cells Electrolytic Cells Chemistry 30 Electrochemistry 21 30-B1.2sts explain that technological problems often require multiple solutions that involve different designs, materials and processes and that have both intended and unintended consequences some processes are used in industry to produce gases, for example: 1. the ___________________________________ for producing _________________________…aluminum oxide is electrolyzed using carbon electrodes…liquid aluminum is collected 2. a _______________________________________________ for producing _____________________________…a saturated sodium chloride solution is electrolyzed…chlorine gas is formed and collected at the anodes Example 1 An electric current is passed through a solution of nickel (II) nitrate using inert electrodes. Predict the anode and cathode reactions, overall reaction, and minimum voltage required. Example 2 An electric current is passed through a solution of potassium iodide using inert electrodes. Predict the anode and cathode reactions, overall reaction, and minimum voltage required. Chemistry 30 Electrochemistry 22 Example 3 An electric current is passed through a solution of copper(II) sulphate using a carbon electrode and a metal electrode. Predict the anode and cathode reactions, overall reaction, and minimum voltage required. 30-B2.4k recognize that predicted reactions do not always occur; eg) the production of chlorine gas from the electrolysis of brine _________________ is an exception to our rules…when water and chlorine are competing as reducing agents, water is the stronger RA but _________________ is chosen because the transfer of e from H2O to O2 is more difficult…called ____________________________ Example 4 An electric current is passed through a solution of sodium chloride. Predict the anode and cathode reactions, overall reaction, and minimum voltage required. Your Assignment: pg 7 “Electrochemical Cells” #1-8 Electrolysis Lab Chemistry 30 Electrochemistry 23 2. Quantitative Study of Electrolysis 30-B2.8k – calculate mass, amounts, current and time in single voltaic and electrolytic cells by applying Faraday’s law and stoichiometry quantitative analysis (stoich) provides information on necessary quantities, current and/or time for electrolytic reactions the unit for charge _________ is the _______________________ one e carries __________________________ of charge this means that one _________of e carry _____________________ of charge ____________________________________ is called the _________________________ (see Data Booklet pg 3) and where: the above equations can be combined into one equation: we can use these equations in stoichiometric calculations for current, time, mass, moles of a substance and moles of e- Example 1 An electrochemical cell caused a 0.0720 mol of e to flow through a wire. Calculate the charge. Chemistry 30 Electrochemistry 24 Example 2 Determine the number of moles of electrons supplied by a dry cell supplying a current of 0.100 A to a radio for 50.0 minutes. Example 3 If a 20.0 A current flows through an electrolytic cell containing molten aluminum oxide for 1.00 hours, what mass of Al() will be deposited at the cathode? Your Assignment: pgs 7-8“Quantitative Study of Electrolysis” #1-17 L. Rust and Corrosion corrosion can be viewed as the process of ______________________ ________________________________________________________ the metal is oxidized causing the loss of ________________________ _____________________________________ most metals develop a thin oxide coating which then _____________ ________________________________________________________ commonly, the oxide coating will scale off leaving new metal exposed an susceptible to corrosion salt will _____________________________________________ by acting as a ________________________________ Chemistry 30 Electrochemistry 25 Cathode: (Reduction) O2(g) + 2H2O(l) + 4e 4 OH(aq) Fe(s) Fe2+(aq) + 2e Anode: (Oxidation) Net: M. Prevention of Corrosion 30-B1.1sts describe the methods and devices used to prevent corrosion; i.e., physical coatings and cathodic protection 30-B2.2sts describe science and technology applications that have developed in response to human and environmental needs apply a coating of _____________to protect metal from oxidation other metals (eg. Zn, Cr, Sn) can be _________________ onto metals that you don’t want to corrode (eg. steel (Fe)) this coating is of a metal that is a ____________________________ than the metal that is to be protected…the coating metal will react instead and is called the ____________________________________ Fe Zn coating this method is also called ___________________________________ has been in use before the science of electrochemistry was developed Sir Humphrey Davy first used cathodic protection on British naval ships in 1824! can be used to protect any metal but steel (iron) is most commonly protected we use steel (iron) for many structures such as buried fuel tanks, septic tanks, pipelines, hulls of ships, bridge supports etc to protect these structures by cathodic protection, an ______________ _________________________________ is connected by a ________ to the structure because the attached metal is a _______________________________ than the iron in the steel, the more active metal supplies the ______________________________________________ and therefore the steel (iron) becomes the cathode and is protected Chemistry 30 Electrochemistry 26 another protection method is alloying pure metals, which changes their ________________________________________ stainless steel contains chromium and nickel, changing steels reduction potential to one characteristic of ______________________ _____________________________________ (basically unreactive) _____________________________ is the process of _____________ ______________________________________________________ by _____________________metal ions in solution an object can be plated by making it the ____________________ in an _____________________________________ containing ions of the plating metal Electrolytic Cell Chemistry 30 Electrochemistry 27