Lesson 4.6 balancing redox reactions
advertisement

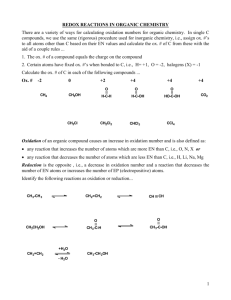
Lesson 4.6 Balancing Redox Reactions Suggested Reading: Zumdahl Chapter 4 Section 4.10. Essential Question: How are redox reactions balanced? Learning Objective: Balance redox reactions. The redox reactions we have discussed so far are relatively simple and can be balanced by inspection without difficulty. However, in the laboratory we often encounter more complex redox reactions involving oxyanions such as chromate (CrO42-), dichromate (Cr2O72-), permanganate (MnO4-), nitrate (NO3-), and sulfate (SO42-). In order to balance these equations we use special techniques that not only help us balance these types of equations, but also give us insight into the electron transfer process. In the In this lesson, you will learn about the half-reaction method (a.k.a. ion-electron method) of balancing redox reactions. In this approach, the overall reaction is divided into two half-reactions, one for oxidation, the other for reduction (Lesson 4.5). You balance each half reaction, then combine them to obtain the overall balanced equation. Half-Reaction Method The half-reaction method differs a little bit depending on whether the the reaction takes place in acidic or basic solution. The prompt usually indicates the type of solution. Steps for Balancing Redox Equations in Acidic Solution Suppose we are asked to balance the equation showing the oxidation of Fe2+ ions to Fe3+ ions by dichromate ions (Cr2O72-) in an acidic solution. The Cr2O72- ions are reduced to Cr3+ ions. The following steps are used to balance equation like these. Step 1: Write the unbalanced equation for the reaction in ionic form. Fe2+ + Cr2O72- → Fe3+ + Cr3+ Step 2: Write the equation in two half reactions using oxidation numbers to identify the oxidation and reduction half-reactions. +2 +3 Oxidation: Fe2+ → Fe3+ +6 +3 Reduction: Cr2O72- → Cr3+ (Note: The oxygen will be dealt with in the next step. Just leave it alone for now.) Step 3: First, balance the atoms in each half-reaction. Second, add H2O to balance the O atoms. Third, add H+ to balance the H atoms. Finally, add electrons to balance the charge. Oxidation: Fe2+ → Fe3+ + e(Note: The atoms were already balanced and there were no O or H atoms to balance. Therefore, all we had to do was add electrons to balance the charge, which is now 2+ on both side of the equation.) The reduction reaction is more complicated. We need to do all of the things mentioned in step 3 in order to balance it. Reduction: 14H+ + Cr2O72- → 2Cr3+ + 7H2O After balancing the Cr and adding H2O to balance the O atoms and H+ to balance the H atoms, we now have 12 positive charges on the left and 6 positive charges on the right (When you add the 14 positive charges on H+ to the two negative charges on Cr2O72- you get 12 positive charges on the left side). Therefore, we must add 6 electrons to the left in order to balance the charges. 14H+ + Cr2O72- + 6e- → 2Cr3+ + 7H2O Step 4: Add the two half-equations together and balance the final equation by inspection. The electrons on both sides must cancel. If the oxidation and reduction half-reactions contain different numbers of electron, we need to multiply one or both half-reactions to equalize the number of electrons. (Note: The first equation was multiplied by 6 to equalize the number of electrons so they could be canceled.) Step 5: Verify that the equation contains the same type and number of atoms and the same changes on both sides of the equation. If you count the atoms you will see they are balanced. The positive and negative charges on each side of the equation must be added and compared to ensure they are in balance. The charges on the left: (12+)+(14+)+(2-) = 24+ The charges on the right: (18+)+(6+)=24+ They balance! (Note: You must carry out step 5 in order to check your work. It is really easy to make mistakes when balancing redox reactions. The number or electrons, H ions or water molecules added can easily be miscalculated. If you find that you are not in balance, walk through the steps one more time. You will usually be able to catch a silly mistake. This step will save you missed points on exams! Steps for Balancing Redox Equations in Basic Solution For reactions in basic medium, we proceed through step 4 as if the reaction were carried out in an acidic solution. Then, for every H+ atom we add and equal number of OH- ions to both sides. Where H+ and OH- ions appear on the same side of the equation, we combine the ions to give H2O. Study Sample Exercise 4.19 on page 165 and Sample Exercise 4.20 on page 167. It would be wise to read this section as well. Students often have difficulty balancing redox equations. The steps presented in you textbook are the same. HOMEWORK: Practice exercises 20.1-20.3