After 2 minutes
advertisement
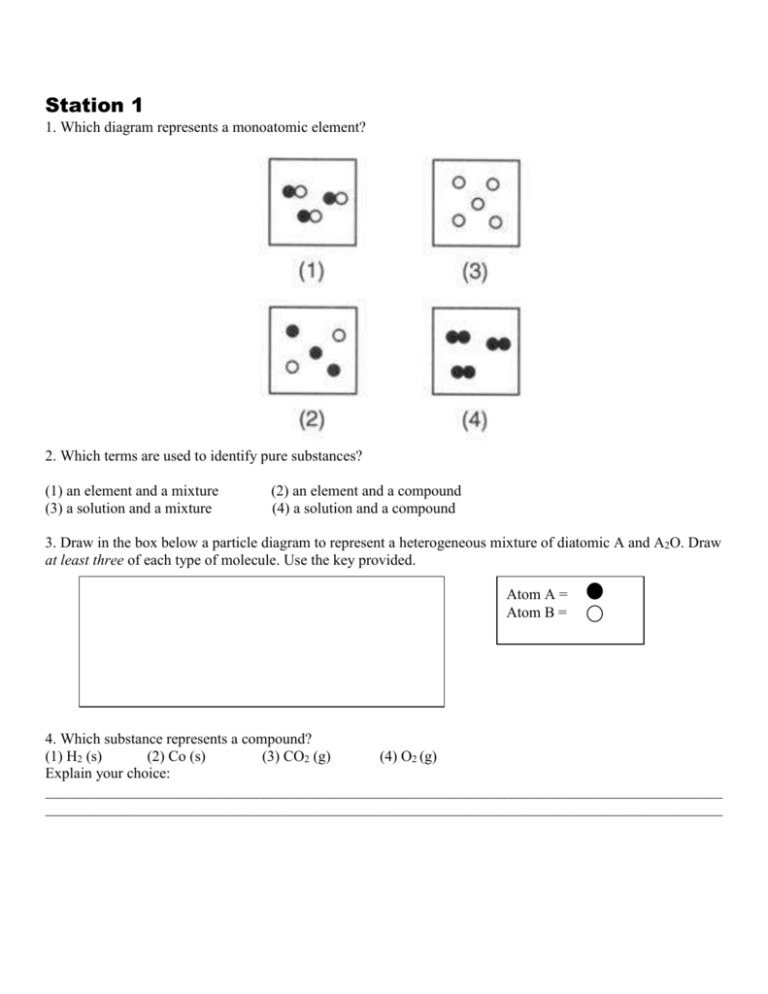
Station 1 1. Which diagram represents a monoatomic element? 2. Which terms are used to identify pure substances? (1) an element and a mixture (3) a solution and a mixture (2) an element and a compound (4) a solution and a compound 3. Draw in the box below a particle diagram to represent a heterogeneous mixture of diatomic A and A2O. Draw at least three of each type of molecule. Use the key provided. Atom A = Atom B = 4. Which substance represents a compound? (1) H2 (s) (2) Co (s) (3) CO2 (g) (4) O2 (g) Explain your choice: __________________________________________________________________________________________ __________________________________________________________________________________________ 5. A dilute aqueous iron (III) nitrate solution is best classified as a (1) homogenous compound (2) homogenous mixture (3) heterogenous compound (4) heterogenous mixture 6. Which particle diagram represents two pure substance? A B Explain your choice: ________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ 7. Complete the diagram below using the terms element, compound, homogeneous and heterogeneous Matter Pure Substance Mixture Station 2 1. Why does the diagram below represent an incorrect particle diagram of a gas? ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ 2. Which describes a substance that is in the solid state? A) It’s particles can flow past one another B) It can be compressed into a smaller volume C) It takes the shape of its container D) Its molecules have a regular geometric pattern. 3. Draw a particle diagram for a solid, liquid and gas in the space below. 4. Which of the following substances will have its atoms in a regular geometric pattern at STP? (1) N2 (2) Fe (3) Hg (4) F2 5. List the three common states of matter in order from the state with the fastest moving particles to the state with the slowest-moving particles. FASTEST ____________ ____________ SLOWEST ______________ In terms of average kinetic energy, explain your choices. __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ 6. Below is a picture of food coloring that has been added to water. Explain why this picture provides evidence to support the idea that water is composed of moving particles After 2 minutes __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ Explain your reasoning for your responses. GOING FURTHER Station 3 of the following pictures as Solid, Liquid, or Gas. 1. Label each Explain your choice. FURTHER 1. CircleGOING if the pictures below show a solid, liquid or gas then circle if the same picture represents an element, a 2. 1.Label each ofof thethe following pictures Element, Compound, orifMixture of Elements, compound or a mixture. Iffollowing you decide it is as a as mixture determine the picture shows a mixture of elements, Label each pictures Solid,then Liquid, or Gas. Explain your choice. Mixture of Compounds, or Mixture of Elements & Compounds. Explain your choice. a mixture of compounds or a mixture of elements and compounds. GOING FURTHER 2. Label each of the Compound, Mixtureyour of Elements, 1. Label each of the following following pictures pictures as as Element, Solid, Liquid, or Gas.orExplain choice. A Mixture of Compounds, or Mixture of Elements & Compounds. Explain your choice. GOING FURTHER E Liquid Solid Gas of Elements, Labeleach eachof ofthe thefollowing followingpictures picturesas as Element, Compound, or Mixture 1.2. Label Solid, Liquid, or Gas. Explain your choice. A Mixture of Compounds, or Mixture of Elements & Compounds. Explain your choice. E 2. Label each of the following pictures asElement Element, Compound, or Mixture of Elements, Compound Mixture of element A Mixture of Compounds, or Mixture of Elements & Compounds. Explain your choice. Mixture of compound E B Mixture of element and compounds A Putting the World in a Box – Student Guide FE B Solid B For each, determine if you think it represents a Solid, Element C del represents anBElement, Compound, or Mixture. GE Compound F Mixture of element Mixture of compound Mixture of element and compounds G C Solid D Gas G F C D C Liquid F Element Liquid G H G Compound H D Gas Mixture of element Mixture of compound Mixture of element and compounds H D Target Inquiry GVSU - 2009, Dale Eizenga, Holland Christian Solid High School Liquid s as Solid, Liquid, or Gas. Explain your choice. H © Target Inquiry 2009-2010 Gas 7 Target Inquiry GVSU - 2009, Dale Eizenga, Holland Christian High School s as Element, Compound, or Mixture of Elements, © Target Inquiry 2009-2010 of Elements & Compounds. Explain your choice. Element Compound Target Inquiry GVSU - 2009, Dale Eizenga, Holland Christian High School © Target Inquiry 2009-2010 E GVSU - 2009, Dale Eizenga, Holland Christian High School Target Inquiry © Target Inquiry 2009-2010 F G 7 Mixture of element Mixture of compound7 Mixture of element and compounds 7 Solid Liquid Gas Element Compound Mixture of element Mixture of compound Mixture of element and compounds 2. Circle the diagram represents a mixture? A 3. B C D Which diagram or diagrams represent a compound of A and B? (A) X, only (B) X and Y (C) Z, only (D) X and Z 4. Explain why this particle diagram does not represent a compound. __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ 5. In Box 1, draw a compound of A and B. In Box 2, draw a mixture of elements made of A and B. In Box 3 draw a mixture of compounds of A and B. 1 2 3 Station 4 1. In an investigation, a dripless wax candle is massed and then lighted. As the candle burns, a small amount of liquid wax forms near the flame. After 10 minutes, the candle’s flame is extinguished and the candle is allowed to cool. The cooled candle is massed. a. Identify one physical change that takes place in this investigation. __________________________________________________________________________________________ __________________________________________________________________________________________ b. Draw a particle diagram showing the change from solid wax to liquid wax. Use BEFORE for particles of wax. AFTER 2. When solid magnesium, Mg combines with oxygen gas, O2, to form solid magnesium oxide, MgO, an intense bright white flash is produced which can result in temporary blindness if looked at directly. ATOM KEY: Physical or Chemical Change? _____________________________ = Mg =O 3. Steam (gaseous water) from a turbine in order to produce electricity. As the gaseous water exits the it condenses into the liquid form. Physical or Chemical Change? _____________________________ ATOM KEY: BEFORE =O AFTER =H turbine








