File
advertisement

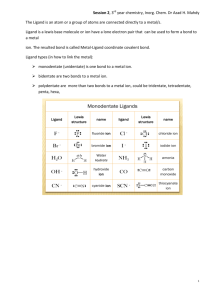
Worksheets on Coloured compounds 1. Suggest why compounds of copper(l) and compounds of scandium(III) are colourless whilst compounds of copper(II) and iron(III) are coloured. _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 2. The hexahydrated iron(III) ion, [Fe(H2O)6]3+, is yellowish brown in colour. Explain (i) why it is coloured and (ii) why it has a different colour to the iron(III) hexacyanide ion, [Fe(CN)6]3-. _____________________________________________________________________________________ _____________________________________________________________________________________ ____________________________________________________________________________________ 3.The thiocyanate ion, SCN- and the hydroxide ion are both monodentate ligands. The thiocyanate ion is higher in the spectrochemical series than the hydroxide ion. (a) Explain what is meant by the term monodentate ligand. _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ (b) What information about the splitting of the d orbitals can be deduced from the fact that the thiocyanate ion is higher in the spectrochemical series than the hydroxide ion? _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 4.Consider the following absorption spectra of two different complex ions of copper(II). Use the colour wheel in Section 17 of the IB data booklet to answer questions (a) and (b) (a) What colour is absorbed by (i) [Cu(H2O)6]2+ and (ii) [Cu(NH3)4(H2O)2]2+ ? ____________________________________________________________________________________ (b) What colour is transmitted by (i) [Cu(H2O)6]2+ and (ii) [Cu(NH3)4(H2O)2]2+ ? ____________________________________________________________________________________ (c) Use the spectra to deduce which is higher in the spectrochemical series, NH3 or H2O. _____________________________________________________________________________________ (d) Explain why [Fe(H2O)6]2+ has a different colour to [Cu(H2O)6]2+ _____________________________________________________________________________________ Answers 1. Copper(I) contain a full 3d sub-level and scandium(III) contains no d electrons so no electronic transitions between split d levels are possible. Copper(II) contains nine d electrons and iron(III) five d electrons. In these compounds the 3d orbitals are split and the energy of the electron transitions between the split levels corresponds to the frequency of light in the visible region. 2. In [Fe(H2O)6]3+ the 3d sub-level is split into two by the water ligands. A d electron can absorb energy as it is promoted from the lower to the higher split level. The colour of the light transmitted is the complementary colour to the light absorbed. In [Fe(CN)6]3- the cyanide ligands cause the amount of splitting to be different so a different wavelength of light is absorbed and the complementary colour transmitted is different to [Fe(H2O)6]3+. 3. (a) Monodentate ligands are Lewis bases which use one non-bonded pair of electrons to form a dative (coordinate) covalent bond with a transition metal or transition metal ion. (b) Thiocyanate ions cause the splitting of the d orbitals to be larger than the splitting by hydroxide ions, i.e. ΔE is greater for SCN–than it is for OH–. 4. (a) (i) [Cu(H2O)6]2+: Red/orange (as the maximum absorption occurs between 600-800 nm). (ii) [Cu(NH3)4(H2O)2]2+: Yellow/orange (as the maximum absorption occurs between 570-620 nm). (b (i) [Cu(H2O)6]2+: Blue/green. (ii) [Cu(NH3)4(H2O)2]2+: Blue/purple. (c) NH3 is higher than H2O as the maximum absorption occurs at lower wavelength (higher energy) so ΔE is greater. (d) Both have the same octahedral shape and the same charge and the metal ion has the same oxidation state. However the metal itself is different (Fe2+ has 26 protons and Cu2+ has 29 protons) so the attraction for the energy levels to the nucleus will be different and the number of d electrons is also different (Fe2+ has six d electrons and Cu2+ has nine d electrons) so the amount the d orbitals are split will also be different.