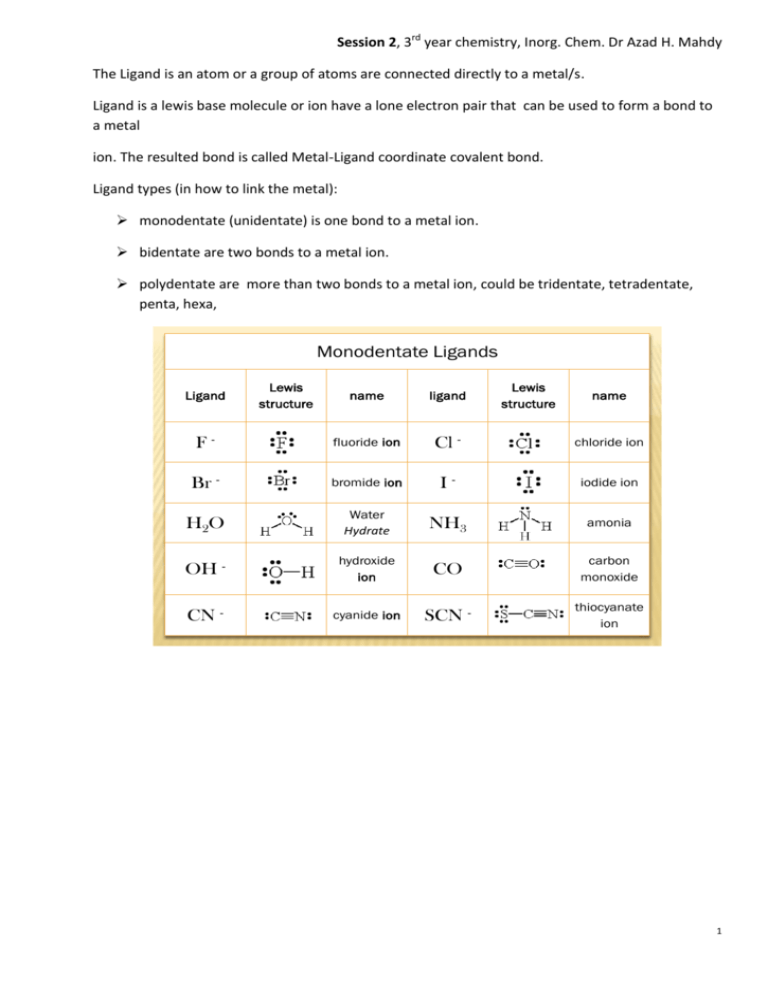
Monodentate Ligands F - Cl - Br - I - H2O NH3 OH - CO CN - SCN -
advertisement
Session 2, 3rd year chemistry, Inorg. Chem. Dr Azad H. Mahdy
The Ligand is an atom or a group of atoms are connected directly to a metal/s.
Ligand is a lewis base molecule or ion have a lone electron pair that can be used to form a bond to
a metal
ion. The resulted bond is called Metal-Ligand coordinate covalent bond.
Ligand types (in how to link the metal):
monodentate (unidentate) is one bond to a metal ion.
bidentate are two bonds to a metal ion.
polydentate are more than two bonds to a metal ion, could be tridentate, tetradentate,
penta, hexa,
Monodentate Ligands
Ligand
Lewis
structure
Lewis
structure
name
ligand
name
F-
fluoride ion
Cl -
chloride ion
Br -
bromide ion
I-
iodide ion
H2O
Water
Hydrate
NH3
amonia
OH -
hydroxide
ion
CO
carbon
monoxide
CN -
cyanide ion
SCN -
thiocyanate
ion
36
1
HOW TO NAME A LIGAND ?
Ligand is a molecule or anion (sometimes cat-ion)
Molecular name differ: water H2O is aqua, amonia NH3 is
ammine when coordinated to the metal.
Anions are named with the same anion name but ending “o”
hydroxyl OH, OH- hydroxo, fluoride F, fluoro F
-CO
Carbonyl
-CO3=
-NH2CH2CH2H2N -
carbonato
ethylenediammine, (en)
-OH- hydroxo
-NH3 ammine -CN- cyano
-NO2
-F- fluoro
-O=
-NO3- nitrato
oxo
-SCN-
nitro
thiocyanato
-Br- bromo
-O2- peroxo
-NC- isocyano
-I- iodo
-SO4= sulfato -NCSisothiocyanato
-ONO- nitrito
-NO
nitrosyl
37
Lewis structure
Naming in coordination compounds
could be long names without spaces.
often, several groups of the ligand are involved in a complex.
The number of ligand molecules per complex is indicated by a Greek prefix: mono- for 1, di(or bi) for 2, tri- for 3, tetra- for 4, penta- for 5, hexa for 6, hepta- for 7, octa- for 8, nona- for
9, deca- for 10.
If the names of ligands already have one of these prefixes, the names are placed in
parenthesis, ( )
2
The prefixes for the number of ligands (already in di name) become bis-, tri becomes tris-,
tetra is tetrakis-, pentakis- ...
For neutral ligands, their normal names are not changed.
Formula of Coordination Compound
Cation then anion
Ligands arranged in alphabetical order before metal ion,
If the ligand is neutral, same molecule name given, with few exceptions.
If the ligand anionic, change the -ide to -o
The prefix indicates number of each.
Oxidation state of metal ion should be in parenthesis ( ) only if more than one possible.
If complex ion was anion, the metal ends with -ate
Total charges of the complex must balance to zero
Complex ion must put in brackets [ ]
K2[Co(NH3)2Cl4]
,
[Co(NH3)4Cl2]Cl
Almost no spaces involved in naming a complex inside brackets [ ]
Spaces are required to name outside the [ ]
Metal ions in complex anions
Iron, Fe
to be
ferrate
Copper , Cu
to be
cuprate
Lead , Pb
to be
plumbate
Silver , Ag
to be
argentate
Gold, Au
to be
aurate
Tin, Sn
to be
stannate
3
The last "e" in names of negative ions are changed to "o" in
names of complexes. Sometimes "ide" is changed to "o“ :
chloride → chloro
-Cl-
-CO3=
-OH- hydroxide → hydroxo
-S= or –S2-
carbonate → carbonato
sulfide → sulfido
-O=
oxide → oxo
-ONO2nitrate → nitrato
(when bonded through O-)
-O2-
peroxide, → peroxo
-NO3nitrate → nitro
(when bonded through N)
-CN-
cyanide → cyano
-SCN-
thiocyanate → thiocyanato-S
(when bonded through S)
-N3-
azide → azido
-NCS-
thiocyanate → thiocyanato-N
(when bonded through N)
-NΞ
or
–N3- nitride → nitrido
-NH2-
amide → amido
42
For big ligands: C5H5N, pyridine,
C5H4N-C5H4N, dipyridyl,
P(C6H5)3, triphenylphosphine.
NH2CH2CH2NHCH2CH2NH2, diethylenetriammine.
Ligands as a group:
HCO3−
hydrogen carbonate
CrO42−
chromate
CO32− carbonate
Cr2O72−
dichromate
H3O+ hydr-oxonium PO43− phosphate
or hydronium
ClO4− perchlorate
HPO42−
hydrogen phosphate
BO33−
borate
AsO43−
arsenate
HSO3−
hydrogen sulfite
MnO4− permanganate
P3−
phosphide
NH4+ ammonium
H2PO4−
dihydrogen phosphate
43
Summary
Name the cation then anion.
Non ionic compounds are given one word name.
Naming ligands first and naming the metal last.
Ligands named in alphabetical order.
Neutral ligands to be named as the same except water (aqua) , amonia (ammine).
Anionic ligands are named by adding “o”, chloride becaomes chloro,..
4
Number of ligands to follow Latin or Greek prefix to indicate how many are present, as: di,
tri, tetra, penta,… if one of these are already there use: bis, tris, tetrakis, pentakis,..
In neutral cationic complex, the name of central metal is followed by its oxidation number in
Roman numeral and put in parenthesis.
In anionic complex, the suffix –ate is added to the name of the central metal.
Examples
Formula
Nomenclature
[Al(OH)4]-
tetrahydroxoaluminumate(III) ion
[Au(CN)2]-
dicyanoaurate(I) ion
[CoBr4]2-
tetrabromocobaltate(II) ion
[AlF6]3-
hexafluoroaluminumate(III) ion
cis-Pt(NH3)2Cl2
cis-diamminedichloroplatinum(II)
[Cu(CN)2]-
dicyanocuprate(I) ion
trans-Cd(NH3)4Cl2
[MoO4]2-
trans-tetraamminedichlorocadmium(II)
permolybdate ion
cis-Pt(NH3)2Cl2
cis-diamminedichloroplatinum(II)
[Ni(CN)4]2-
tetracyanonickelate(II) ion
[Cd(CN)4]2-
tetracyanocadmiumate(II) ion
cis-Cd(NH3)4Cl2
cis-tetraamminedichlorocadmium(II)
[MnCl6]4-
hexachloromanganate(II) ion
[FeCl4]-
tetrachloroferrate(III) ion
Na3[Co(CO3)3].3H2O
[Co(NH3)5(CO3)] NO3
[Cr(C5H7O2)3]
sodium tricarbonatocobalt(III) trihydrate
pentaamminecarbonatocobalt(III) nitrate
trisacetylacetonechromium(III)
K4[Co2(C2O4)4(OH)2].3H2O
potassium tetraoxalatodi-μ-hydroxocobalt(III) trihydrate
K2 [PtCl4]
potassium tetrachloroplatinate(II)
[PtCl4]2-
tetrachloroplatinate(II) ion
[Co(en)2Cl2] Cl
dichlorobis(ethylenediamine)cobalt(III) chloride
5
Nomenclature
Formula
potassium hexacyanoferrate(II)
K4 [Fe(CN)6]
tetraamminecuprate(II) sulfate
[Cu(NH3)4] SO4
amminetrichloroplatinate(II) ion
[Pt(NH3)Cl3]-
pentaaquachlorochromium(III) sulfate
[Cr(H2O)5Cl] SO4
triamminetrichlorochromium(III)
[ Cr(NH3)3Cl3]
chloro(ethylene)dihydridobis(triphenylphosphinerhodium(III)
tetraaquadichloromolybdate(III) chloride
[Rh(PPh3)2(C2H2)ClH2]
[Mo(H2O)4Cl2] Cl
tetraaquadichloromolybdate(III) ion
[Mo(H2O)4Cl2]+
hexaamminechromium(III) hexachloroiridate(III)
[Cr(NH3)6 ][IrCl6]
[Cr(H2O)2 BrClFI] -
diaquabromochlorofluoroiodochromium(III) ion
tetraammineaquafluorocobalt(III) ion
[Co(NH3)4(H2O)F] 2+
bis(ethylenediammine)cuprate(II) ion
[Cu(en)2] 2+
bis(ethylenediammine)cuprate(II) tetrachloroplatinate(II)
[Cu(en)2][PtCl4]
QUIZ
1. Write the nomenclature for the formula:
Br
[Sr(py)5Br2]
dibromopentapyridinestrontium(II) py
[Be(OH)4]2tetrahydroxoberylium(II) ion
py
py
Sr
py
py
Br
2. Find the molecular formula for the following compounds:
hexaaquamagnesium(II) perchlorate
[Mg(H2O)6] ClO4
Fe
trisacetylacetonatoferrate(III)
[Fe(acac)3]
tetracarbonylruthenium(II) ion
[Ru(CO)4]2O
O
O
O
O
O
6
3. Write the nomenclature for the formula:
[Rh(PPh3)3Cl]
chlorotris(triphenylphosphine)rhodium(I)
[Cr(H2O)5Cl]2+
pentaaquachlorochromium(III) ion
4. Find the molecular formula for the following compounds:
tetraamminedichlorocobalt{(III) ion
[Co(NH3)4Cl2]+
tetraammineargentate(I) ion
[Ag(NH3)4]+
hexaaquaferrate(III) ion
[Fe(H2O)6]3+
Reactions of coordination compounds
How can we prepare coordination compounds or convert them into other compounds? The answer
is by a
Redox reaction, Two possible mechanisms:
Outer sphere: coordination sphere remain intact.
Inner sphere: ligands transfer between coordination spheres.
OUTER SPHERE
[Fe(CN)6]4- + [ IrCl6]2- → [Fe(CN)6]3- + [ IrCl6]3The coordination spheres stays the same around each metal;
reaction involves only transfer of electrons .
53
In a self-exchange reaction, the left-hand and right-hand sides of the equation are identical; only
electron transfer, and no net chemical reaction, takes place.
7
[Co(Phen)3]2+ + [Co(Phen)3]3+ → [Co(Phen)3]3+ + [Co(Phen)3]2+
For the net chemical reaction can be seen in:
[Fe(CN)6]4- + [Fe(Phen)3]3+ → [Fe(CN)6]3- + [Fe(Phen)3]2+
When the reactants have different bond lengths, vibrationally excited states with equal bond length
must be formed in order to allow electron transfer.
overall
reaction
Co2+
Co3+
Co3+
Co2+
Electron
transfer
(Co2+)*
(Co3+)*
(Co3+)*
(Co2+)*
Inner sphere
[Co(NH3)5Cl]2+ + [Cr(H2O)6]2+ + 5H2O → [Co(H2O)6]2+ + [Cr(H2O)5Cl]2+ + 5NH3
Cl- ligand been transferred from Co to Cr
[Co(NH3)5CN]2+ + [Cr(H2O)6]2+ + 5H2O fast →
[Cr(H2O)5NC]2+ in 30seconds
→
[Co(H2O)6]2+ + [Cr(H2O)5NC]2+ + 5NH3
[Cr(H2O)5CN]2+
8
56
Chelate
Monodentate: attached ligand to only one metal.
polydentate: attached at two or more separated metals.
Examples:
ethylenediamine (en) NH2CH2CH2NH2 attached by its two nitrogen atoms.
Ethylenediaminetetraacetic acid, EDTA
upto six positions.
(HOOCCH3)2NCH2CH2N(CH3COOH)2 attached to
9
2-
Chelating Ligands
O
C
O
O
H2C
acetate ion
carbonyl
O
CH2
H2N
O
O
O
N
O2C
NH2
M
O2C
O
O
N
O2C
acetyleacetanato, acac
O2C
M
ethylenediammine, en
N
ethylenediamminetetraacetate, EDTA
O
N
oxalate, ox
C2O42-
M
M
N
O
O
O
Na+
O
N
O
N
bipyridine
(bipy)
N
M
O
O
phenanthroline, phen
Na+ [18-crown-6]
Chelate effect
Chelating ligands give much larger values of formation constant, kf
[Ni(H2O)6]2+ + 6NH3 ↔ [Ni(NH3)6]2+ + 6H2O
[Ni(H2O)6]2+ + 3 en ↔ [Ni(en)3]2+ + 6H2O
kf=4x108
kf=2x1018
sequestering agents are chelating agents that used to remove unwanted metals.
in medicine, sequestering agents are used to selectively remove toxic metal ions e.g. Hg2+
and Pb2+ while leaving biologically important metals
Chelating Ligands
One of an important chelating agent is ethylenediamminetetraacetate, (EDTA) 4EDTA is used to
Tie up Ca2+ in bathroom cleaners, shower sprays
prevent blood clots
remove heavy metals from the body when poisoned
solubilize iron in plant fertilizer
remove iron taste from mayonnaise (arising from its preparation in iron vats)
10
METAL CHELATE IN LIVING SYSTEMS
CH2
Chlorophyll is the
green part of
plants involved in
photosynthesis.
it is a
magnesium(II)
complex ion.
CH3
H
CH
CH2CH3
H3C
N
N
Mg
HC
CH
N
N
H
CH3
H3C
H
H2C
H
CH3
O
COOCH3
63
METAL CHELATE IN LIVING SYSTEMS
The haem unit in haemolglobin of our blood involves a
rigid chelating ligand.
H2O, O2 or CO can be the sixth coordination ligand.
63
11









