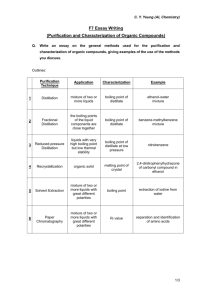
org synthesis
advertisement
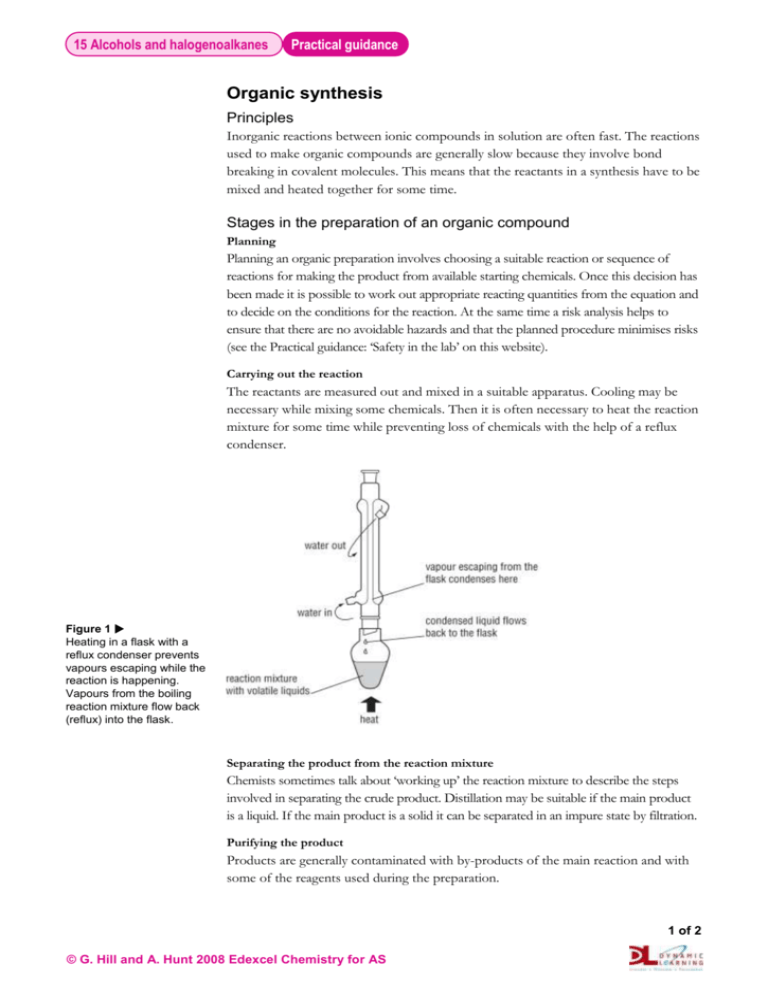
15 Alcohols and halogenoalkanes Practical guidance Organic synthesis Principles Inorganic reactions between ionic compounds in solution are often fast. The reactions used to make organic compounds are generally slow because they involve bond breaking in covalent molecules. This means that the reactants in a synthesis have to be mixed and heated together for some time. Stages in the preparation of an organic compound Planning Planning an organic preparation involves choosing a suitable reaction or sequence of reactions for making the product from available starting chemicals. Once this decision has been made it is possible to work out appropriate reacting quantities from the equation and to decide on the conditions for the reaction. At the same time a risk analysis helps to ensure that there are no avoidable hazards and that the planned procedure minimises risks (see the Practical guidance: ‘Safety in the lab’ on this website). Carrying out the reaction The reactants are measured out and mixed in a suitable apparatus. Cooling may be necessary while mixing some chemicals. Then it is often necessary to heat the reaction mixture for some time while preventing loss of chemicals with the help of a reflux condenser. Figure 1 Heating in a flask with a reflux condenser prevents vapours escaping while the reaction is happening. Vapours from the boiling reaction mixture flow back (reflux) into the flask. Separating the product from the reaction mixture Chemists sometimes talk about ‘working up’ the reaction mixture to describe the steps involved in separating the crude product. Distillation may be suitable if the main product is a liquid. If the main product is a solid it can be separated in an impure state by filtration. Purifying the product Products are generally contaminated with by-products of the main reaction and with some of the reagents used during the preparation. 1 of 2 © G. Hill and A. Hunt 2008 Edexcel Chemistry for AS 15 Alcohols and halogenoalkanes Practical guidance A liquid product can be purified by shaking in a tap funnel with reagents that do not mix with the product but which can extract the impurities. Then drying and distillation gives a pure product. Figure 2 If the organic product is a liquid which does not mix with water it can be partly purified by shaking with aqueous reagents in a tap funnel. Checking the identity, purity and yield of the product The final distillation of a liquid provides clues to the identity and purity of the product. During the distillation the main fraction should distil off over a narrow temperature range at the boiling temperature of the liquid. Infrared spectroscopy is a quick and convenient way to check the identity and purity of any organic product. The spectrum of the product can be checked against a definitive spectrum in a database (see Topic 17). Measuring the melting temperature of a solid helps to check whether or not it is pure and to confirm its identity. Pure solids melt at a sharp temperature. Another useful technique for checking the purity of a solid is thin-layer chromatography. A pure compound produces just one spot on the developed chromatogram. 2 of 2 © G. Hill and A. Hunt 2008 Edexcel Chemistry for AS