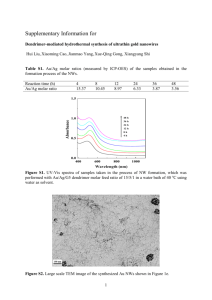
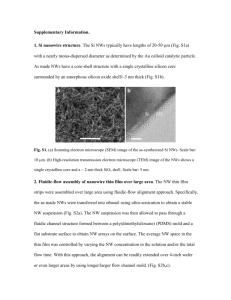
Supplementary Data Figure Captions
advertisement

Supplementary Data Figure Captions Fig. S1 XRD of S-TiO2 NWs calcined at different temperature Fig. S2 SEM images of S-TiO2 NWs Fig. S3 HRTEM and SAED (inset) of S-TiO2 NWs calcined at 600 oC Fig. S4 XPS of S-TiO2 NWs and NS-TiO2 NWs (a) S 2p; (b) N 1s; (c)Ti 2p;(d) O 1s Fig. S5 BET of (a) NS-TiO2 NPs, (b) S-TiO2 NWs, and (c) NS-TiO2 NWs Fig. S6 UV-vis spectrum of doped and undoped TiO2 NWs Fig. S1 (A.U.) 9000 Intensity 10000 6000 A - a n a t a s e B - T 2i( O B ) 8000 S - T2 i NO W s - 5 0 0 S - T2 i NO W s - 6 0 0 S - T2 i NO W s - 7 0 0 A ( 1 0 1 ) 7000 A ( 0 0 4 ) 5000 A ( 2 1 1 ) A ( 2 0 0 ) A ( 2 0 4 ) 4000 A ( 1 0 5 ) 3000 A ( 1 0 3A ) ( 1 1 2 ) 2000 B ( 0 0 3 ) 1000 0 10 15 20 25 30 35 B 40 45 degree(2θ) 50 55 60 65 70 Fig. S2 Fig. S3 Intensity/A.U. Fig. S4 S 2p 1 6 4 . 6 N S - T2 iNOW s 1 6 4 . 7 S - T 2i O N W s 170 168 166 164 162 Binding 160 158 energy/eV (a) Intensity/A.U. N 408 1s N S - T2 iNOW s 397.5 406 404 402 400 398 Binding 396 394 392 energy/eV 390 (b) 456.2 Ti 2p Intensity/A.U. N S - T2 i N OW s 462.0 456.3 S - T 2i O NWs 462.0 466 464 462 460 458 456 Binding 454 452 450 energy/eV (c) O 1s 527.5 527.4 Intensity/A.U. NS-TiO2 NWs 529.4 527.5 527.4 S-TiO2 NWs 529.2 532 531 530 529 528 Binding energy/eV (d) 527 526 525 Fig. S5 70 0 . 0 0 0 7 d V / d / Dg (, cn m m ) 3 60 50 40 Adsorbed 0 . 0 0 0 6 0 . 0 0 0 5 0 . 0 0 0 4 3 , Se T( P ) v o l/ ug m cm 80 0 . 0 0 0 3 0 . 0 0 0 2 0 . 0 0 0 1 0 . 0 0 0 0 30 10 20 30 40 50 60 P o r e 70 80 90 1 0 0 d i a m e t e r ( n m ) 20 10 0 0 . 0 0 . 2 0 . 4 0 . 6 Relative 0 . 8 1 . 0 p r e 0s) s u r e ( P / P (a) 90 0.0007 0.0006 70 0.0005 dV/dD(cm /g,nm) 60 0.0004 3 3 Adsorbed volume(cm /g,STP) 80 50 0.0003 0.0002 0.0001 40 0.0000 10 30 20 30 40 50 60 70 80 90 100 Pore diameter(nm) 20 10 0 0.0 0.2 0.4 0.6 Relative Pressure(P/P0) 0.8 1.0 (b) 80 0 .0 0 0 7 70 60 0 .0 0 0 5 0 .0 0 0 4 3 d V /d D (cm/g ,n m ) 3 Adsorbed volume(cm /g,STP) 0 .0 0 0 6 50 40 0 .0 0 0 3 0 .0 0 0 2 0 .0 0 0 1 0 .0 0 0 0 30 10 20 30 40 50 60 70 80 90 100 X A xis T itle 20 10 0 0.0 0.2 0.4 0.6 0.8 1.0 Relative pressure(P/P0) (c) The surface area and porosity distribution of NS-TiO2 NPs, S-TiO2 NWs and NS-TiO2 NWs calcined at 500 oC were investigated using nitrogen adsorption and desorption isotherms. The isotherms of these three photocatalysts are typical type IV-like with a type H2 hysteretic loop, which indicates the presence of mesoporous materials. The plot of the pore size distribution (inset) was determined by using the Barrett-Joyner-Halenda (BJH) method from the desorption branch of the isotherm. It shows that these three photocatalysts clearly have mesoporous structure. The average pore diameter of NS-TiO2 NPs is about 25 nm and the BET surface area is 29.1 m2 g−1. The average pore diameter and the BET surface area of S-TiO2 NWs and NS-TiO2 NWs are about 35 nm, 22 nm and 39.8 m2 g−1, 35.1 m2 g−1, respectively. Fig. S6 Absorbance(A.U.) 1.2 0.9 TiO2 NWs S-TiO2 NWs NS-TiO2 NWs 0.6 0.3 0.0 300 400 500 600 700 Wavelength(nm) Fig. S6 exhibits the UV-vis absorption spectra of S-TiO2 NWs and NS-TiO2 NWs calcined at 600 oC compared to TiO2 NWs. A noticeable shift of the optical absorption edges of the doped TiO2 toward the visible region of the solar spectrum was observed whereas pure TiO2 NWs has no ability to respond to visible light. Obviously, this shift towards the longer wavelengths originates from the band gap narrowing of titanium dioxide by sulfur doping or nitrogen/sulfur codoping.