Group 5

Name(s): ___________________________________ Period: _________ Group: ________
Lab: DENATURING PROTEINS—Group 5
Objective: To experiment with different methods of denaturing the protein found in egg white (albumin) and milk (casein)
Background:
Proteins are large molecules made up of small amino acids. Proteins are held in a natural shape due to the interaction of side groups on the amino acids from one part of the molecule to another area of the molecule. These interactions may be hydrogen bonds or disulfide bonds. We can denature the proteins by disrupting the H-bonds that are within the structure. When this happens the overall shape of the protein changes and new properties can be observed. The shape of a protein is associated with food processing properties, such as solubility, gel formation, and enzyme activity.
PROTEIN DENATURATION or WHAT HAPPENS WHEN YOU FRY AN EGG?
In the egg whites the albumin will change from clear to white. We will explore how the following denature egg albumin as well as milk casein.
When egg white is heated above a certain temperature (i.e. 60 °C) bonds in the protein molecules break and reform in different ways. This causes the protein molecules to denature or lose their characteristic three-dimensional structure. As the proteins uncoil, they tend to interact with each other to form a solid mass or coagulum (the process called coagulation). This explains the changes that we observe as we watch an egg being cooked. However, factors other than high temperature may also produce this denaturation phenomenon.
Heat – done by cooking
Acids & bases – can form ions on some side groups of amino acids
Organic compounds – form their own hydrogen bonds with the amino acids
Heavy metals – react with disulfide bonds
Procedures for Denaturation Labs:
Group 5 – Denaturation by ORGANIC SOLVENT (CH
3
-CH(OH)-CH3, Rubbing Alcohol-Isopropyl
Alcohol)
Materials
1 glass dish
1 paper cup
1 egg
5 ml of CH
3
-CH(OH)-CH3 (Rubbing Alcohol-Isopropyl Alcohol)
2 –50 ml beakers of milk (15 ml each)
50 ml beaker of Lemon juice (5 ml)
2- Stirring rods
1
Egg Albumin Procedure:
1.
Separate 1 egg white, placing the egg whites in a large glass dish. Discard the egg yolk into the paper cup.
Note: The clarity of the egg white this is your baseline or control, record your
observations in Data Table 1 below.
2.
Pour (5ml) rubbing alcohol into the dish containing an egg white and stir.
3.
Record your observation in the Egg Albumin Data Table (Data Table 1) below, and for
Group 5 and on the front board.
4.
Now Do Milk (Casein) Denaturation.
Procedure for Milk (Casein) Denaturation:
1.
Pour the 5 ml of Lemon juice into one of the beakers of milk and stir.
2.
Compare the beaker with lemon juice and milk to the beaker with only milk. Look for any changes in color, texture, etc…
3.
Record Observations in Data Table 2 on page 3, and on the front board next to your group number.
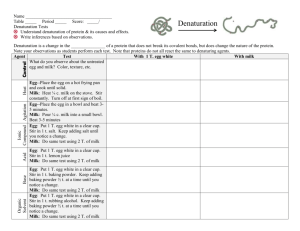
DATA TABLE 1~ Egg Albumin Data Table
Group
ALL
GROUPS
1
2
3
4
5
6
Added
Nothing—initial observation
Heat
NaCl – Ionic
Compound (heavy metal)
NaHCO
3
– Base
Lemon Juice – Acid
Rubbing Alcohol –
Organic Liquid
Pine Apple Juice-
Acid
Observations
2
DATA TABLE 2~ Milk Casein Data Table
Group Added
Your group Lemon Juice – Acid
Observations
Other groups
Lemon Juice – Acid
Post Lab Questions:
1. Copy and record all the Data on the front board into your data tables.
2. What three factors have caused denaturation of the egg albumin in this lab?
3. What common result occurred in all of the experiments with the egg albumin?
4. What results did you get with the milk casein?
3
5. Was the milk casein denatured?
6. Did all of the groups get the same results with the milk casein? If not list the differences.
7. Explain the changes that you observed in terms of change in protein structure at the
molecular level (be very specific). You may want to get a copy of an article called
‘Denaturation’ from your instructor to help you answer this question.
4