Water and pH SG KEY
advertisement
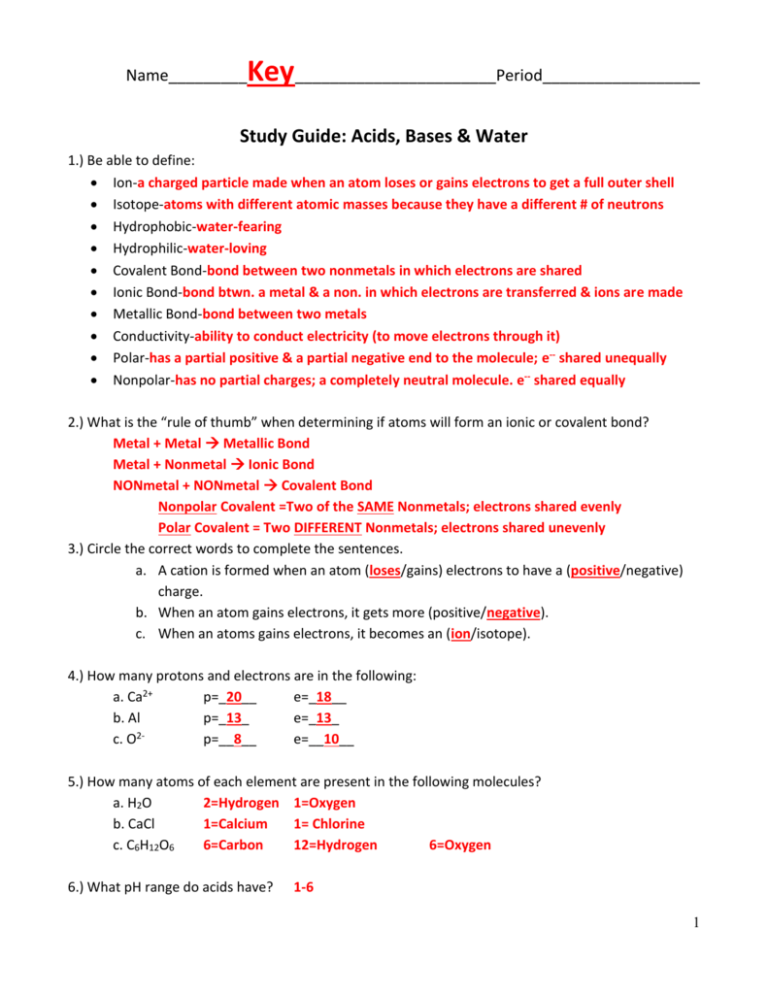
Key_______________________Period__________________ Name_________ Study Guide: Acids, Bases & Water 1.) Be able to define: Ion-a charged particle made when an atom loses or gains electrons to get a full outer shell Isotope-atoms with different atomic masses because they have a different # of neutrons Hydrophobic-water-fearing Hydrophilic-water-loving Covalent Bond-bond between two nonmetals in which electrons are shared Ionic Bond-bond btwn. a metal & a non. in which electrons are transferred & ions are made Metallic Bond-bond between two metals Conductivity-ability to conduct electricity (to move electrons through it) Polar-has a partial positive & a partial negative end to the molecule; e-- shared unequally Nonpolar-has no partial charges; a completely neutral molecule. e-- shared equally 2.) What is the “rule of thumb” when determining if atoms will form an ionic or covalent bond? Metal + Metal Metallic Bond Metal + Nonmetal Ionic Bond NONmetal + NONmetal Covalent Bond Nonpolar Covalent =Two of the SAME Nonmetals; electrons shared evenly Polar Covalent = Two DIFFERENT Nonmetals; electrons shared unevenly 3.) Circle the correct words to complete the sentences. a. A cation is formed when an atom (loses/gains) electrons to have a (positive/negative) charge. b. When an atom gains electrons, it gets more (positive/negative). c. When an atoms gains electrons, it becomes an (ion/isotope). 4.) How many protons and electrons are in the following: a. Ca2+ p=_20__ e=_18__ b. Al p=_13_ e=_13_ 2c. O p=__8__ e=__10__ 5.) How many atoms of each element are present in the following molecules? a. H2O 2=Hydrogen 1=Oxygen b. CaCl 1=Calcium 1= Chlorine c. C6H12O6 6=Carbon 12=Hydrogen 6=Oxygen 6.) What pH range do acids have? 1-6 1 7.) What pH range do bases have? 8-14 8.) What pH do neutral solutions have? 7 9.) Describe the taste of: a.) Acids: Sour b.) Bases: Bitter & Soapy 10.) What ion does an acid produce? __Hydronium [H3O]+__ or __Hydrogen ion [H]+_ 11.) What ion does a base produce? ___Hydroxide [OH]-_ 12.) A pH of 4 is more or less acidic than a pH of 6? __MORE Acidic__ 13.) If something has a pH of 3 and you add something that is a base to it, what will happen to the pH? Neutralization will occur. The acid will become more neutral, therefore the pH scale number will increase. 14.) How do the acid and base ions compare at a pH of 9? More Hydroxide [OH]- ions are in the solution than Hydrogen [H]+ ions. 15.) How do the acid and base ions compare at a pH of 7? The Hydrogen ions [H]+ equal the amount of Hydroxide ions [OH]-. 16.) pH Scale: Measures the ____Hydrogen ions [H]+___ ion concentration. 17.) Fill in the blanks of this pH Scale (make sure to label the acid side and base side): [H]+ > Acids [OH]- [H]+ < [OH]- Bases pH:_1_ pH:_7_ pH: 14 Strong Acid Neutral Strong Base 18.) If a solution has a higher concentration of the acidic ion than the basic ion it is considered to be a(n) ___Acid___. 19.) If a solution has a higher concentration of the basic ion than the acidic ion, it is considered to be a(n) __Base___. 20.) If a solution has an equal concentration of the acidic ion and basic ion it is considered to be a(n) _Neutral_. 2 21.) Compare strong acids to weak acids and include the following in your comparison: Ionize, electrolyte, pH. Ionize completely Strong electrolytes pH closer to 1 Do not ionize completely Weak Electrolytes pH closer to 6 22.) Compare strong bases to weak bases and include the following in your comparison: Ionize, electrolyte, pH. ionize into a metal cation and an OHStrong electrolytes pH closer to 14 make OH- ions by combining with water weak electrolytes pH closer to 8 23.) What color does litmus paper change to when placed in a base? _Blue__ 24.) What color does litmus paper change to when placed in an acid? __Red___ 25.) What is an electrolyte? A substance that can conduct electricity (allows electrons to flow through it). 26.) What is neutralization? What so the acidic and basic ion combine to form? A reaction between a base and an acid to form a salt and water. Hydronium [H 3O]+donates an H+ to [OH]- to make H2O. 27.) Baking soda is a base and vinegar is an acid. What type of chemical reaction occurred when these were combined? Neutralliztion 28.) Explain the phrase “like dissolves like”. Be sure to discuss polar vs. nonpolar and hydrophobic vs. hydrophilic. Polar molecules can dissolve in polar solvents like water, but nonpolar molecules will not. They can only be dissolved in nonpolar solvents like oil. This is why we call polar molecules HYDROPHILIC because they are “water-loving” and nonpolar molecules HYDROPHOBIC because they are “water-fearing”. 3 29.) Explain why water is said to be polar. 30.) Draw two water molecules. Show how they would interact with each other. How would they arrange themselves? Label the following bonds: Hydrogen bond, polar covalent bond. Water molecules arrange themselves to keep the oxygens as far away from each other as possible, while keeping the Hydrogens near the Oxygens (The Oxygen’s are attracted to the Hydrogens) 4