Supporting Information for Study on the surface energies and
advertisement

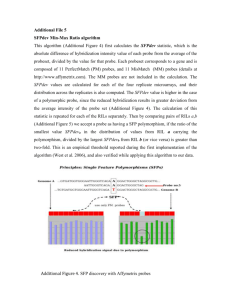
Supporting Information for Study on the surface energies and dispersibility of graphene oxide and its derivatives Jinfeng dai, Guojian Wang, Lang Ma, Chengken Wu Further experimental details IGC measurements and theory: For the determination of thermodynamic surface parameters, the net retention volume Vn is computed according to Eq. (1):[1, 2] 𝑃0 −𝑃𝑤 𝑉𝑛 = 𝐹﹒𝑗﹒(𝑡𝑟 − 𝑡0 )﹒ ( 𝑃0 ) ﹒ (𝑇 𝑇𝑐 𝑚𝑒𝑡𝑒𝑟 ) 3 (𝑃 ⁄𝑃 )2 −1 𝑗 = 2 [(𝑃𝑖 ⁄𝑃0 )3 −1] 𝑖 0 (1) (2) Where F is the flow rate, tr and t0 are the retention and dead times respectively, p0 is the pressure at the flow meter, pw is the vapor pressure of water at the temperature of the flow meter (Tmeter), and the Tc is the column temperature. j is the James-Martin compressibility factor for the correction of gas compressibility when the column inlet (pi) and outlet (p0) pressure are different and it is given by Eq. (2)[1, 2] According to Fowkers[3], the surface free energy of solid, 𝛾𝑠𝑡 , which can be considered to be a sum of a dispersive component, 𝛾𝑠𝑑 , due to van der Waals interactions, and a specific component, 𝛾𝑠𝑃 , such as acid–base interactions, hydrogen-bonding, or π-π stacking. The 𝛾𝑠𝑑 value of stationary phase is measured when n-alkanes are used as probes, and can be obtained according to Dorris and Gray method[4]. Where 𝑅𝑇 · ln 𝑉 for n-alkanes is plotted against the number of carbon atoms of the probe, the 𝛥𝐺𝐶𝐻2 can be determined from the slope of the resulting line. Then, the 𝛾𝑠𝑑 can be calculated using the following Eq. (3), as seen in Figure S2: γ𝑑𝑠 = (𝛥𝐺𝐶𝐻2 ) 2 4𝛾𝐶𝐻2 ·(𝑎𝐶𝐻2 ·𝑁𝐴 ) 2 (3) Where 𝑁𝐴 is the Avogadro constant, 𝑎𝐶𝐻2 is the area of a –CH2– group (0.06 nm2) and 𝛾𝐶𝐻2 the surface energy of a solid consisting of only –CH2– groups. The value of 𝛾𝐶𝐻2 with temperature can be obtained by Eq. (4): 𝛾𝐶𝐻2 (𝑚𝐽 · 𝑚2 ) = −0.058(𝑚𝐽 · 𝑚2 ) × 𝑇 + 52.6(𝑚𝐽 · 𝑚2 ) (4) If the polar probes are injected, both dispersive and specific interactions are established with the solid surface, ∆𝐺, being the adsorption free energy, defined by Eq. (5)[2, 5, 6]: ∆𝐺 = ∆𝐺 𝑑 + ∆𝐺 𝑠𝑝 (5) Where ∆𝐺 𝑑 is the adsorption free energy of dispersive interaction; While ∆𝐺 𝑠𝑝 is the specific interaction contributions to ∆𝐺 which reflects specific interaction (such as acid–base interactions, hydrogen-bonding, or π-π stacking) between chemical surface and probes. The value of ∆𝐺 𝑠𝑝 is difficult to obtain through Dorris and Gray method. however, 𝑅𝑇 · ln 𝑉 can be plotted against the molecular polarizabilities (PD) of the probes according to an approach defined by Dong et al.[7] The value of ∆𝐺 𝑠𝑝 results from the distance between the 𝑅𝑇﹒ ln 𝑉 value of polar probe and the straight n-alkanes line, as shown in Figure S3. From these ∆𝐺 𝑠𝑝 values, polar surface energies of solid (𝛾𝑠𝑃 ) are calculated using the following Eq. (6)[8, 9] based on the theory of Good-Van Oss[10, 11]: ∆𝐺 𝑠𝑝 = 2﹒𝑁𝐴 ﹒𝑎﹒((𝛾𝐿+ 𝛾𝑆− )1⁄2 + (𝛾𝐿− 𝛾𝑆+ )1⁄2 ) (6) Where 𝛾𝑆+ and 𝛾𝑆− are the acidic and basic parameters of the solid surface, respectively, and 𝛾𝐿+ and 𝛾𝐿− are the acidic and basic parameters of the probe molecules, respectively. In our work, we adopted DCM and EtAc as a monopolar acidic probe and a monopolar basic probe, and their acidic and basic parameters − + + (using 𝛾𝐷𝐶𝑀 as 0.0 mJ·m-2 and 𝛾𝐷𝐶𝑀 as 5.20 mJ·m-2; assuming 𝛾𝐸𝑡𝐴𝑐 to be 0.0 − mJ·m-2 and 𝛾𝐸𝑡𝐴𝑐 to be 19.2 mJ·m-2) are provided. Consequently, the 𝛾𝑠𝑃 is determined according to Eq. (7)[10, 11]: 𝛾𝑠𝑃 = 2(𝛾𝑠+ 𝛾𝑠− ) 1⁄ 2 (7) Once the 𝛾𝑠𝐷 and 𝛾𝑠𝑃 are obtained, the total surface energy (𝛾𝑠𝑇 ) is calculated as following Eq. (8): 𝛾𝑠𝑇 = 𝛾𝑠𝐷 + 𝛾𝑠𝑃 (8) Tables Table S1 The physicochemical properties of probes 𝛂 PD AN DN Specific (Å)2 (cm3﹒mol-1)* (kJ﹒mol-1) (kJ﹒mol-1) Character C6 51.5 29.9 - - neutral C7 57.0 34.6 - - neutral C8 62.8 39.2 - - neutral C9 69.0 43.8 - - neutral DCM 31.5 16.4 16.4 0 acidic EtAc 48.0 22.2 6.3 71.7 amphoteric (stongly basic) THF 45.0 20.0 2.1 84.4 basic Probe * Molecular Deformation Polarizability Table S2 EA, XPS data of samples Sample Element EA (wt %) XPS (at %) C 65.14 68.6 O 33.35 31.0 N 0.17 0.4 C/O 2.60 2.21 C 68.94 72.4 O 29.54 27.6 C/O 3.11 2.62 C 79.87 83.3 O 19.36 16.7 C/O 5.50 4.98 C 91.56 90.6 O 6.91 8.2 N 1.34 1.2 C/O 17.67 11.0 GO COOH-GO EG-rGO rGO Table S3 Quantitative comparison of C1s and O1s peaks for samples C 1s (%) O 1s (%) Sample sp2/284.6eV C-O/286.3eV C=O/288.1eV O-C/533.2eV O=C/531.5eV GO 41.5 45.4 13.1 70.9 29.1 COOH-GO 49.8 33.8 16.4 41.6 58.4 EG-rGO 67.8 23.6 8.6 59.4 40.6 rGO 72.8 7.1 3.4 48.9 51.1 Figures Fig. S1 High resolution O1s XPS spectra of GO, COOH-GO, EG-rGO and rGO Fig. S2 The plot of 𝑅𝑇 · ln 𝑉 Vs. carbon number Fig. S3 Determination of ∆𝐺 𝑠𝑝 for polar probes by Dong`s method Fig. S4 Dispersion states of GO, COOH-GO, EG-rGO and rGO versus the dispersive components (δD) of solvents Fig. S5. AFM images of GO/DMF, COOH-GO/DMF, EG-rGO/NMP and rGO/NMP References 1. Conder JR, Young. CL (1979) Physicochemical measurement by gas chromatography. John Wiley and Sons: Chichester. 2. Santos JMRCA, Guthrie JT (2005) Study of a core-shell type impact modifier by inverse gas chromatography. J Chromatogr A 1070(1-2): 147-154. 3. Fowkes FM (1964) Attractive forces at interfaces. Industrial & Engineering Chemistry 56(12): 40-52. 4. Dorris GM, Gray DG (1980) Adsorption of n-alkanes at zero surface coverage on cellulose paper and wood fibers. J Colloid Interface Sci 77(2): 353-362. 5. Laub RJ (1980) Physicochemical measurement by gas chromatography J High Resolut Chromatogr 3(9): 486-486. 6. Santos JMRCA, Fagelman K, Guthrie JT (2002) Characterisation of the surface Lewis acid-base properties of poly(butylene terephthalate) by inverse gas chromatography. J Chromatogr A 969(1-2): 111-118. 7. Dong S, Brendlé M, Donnet JB (1989) Study of solid surface polarity by inverse gas chromatography at infinite dilution. Chromatographia 28(9-10): 469-472. 8. Traini D, Young P, Thielmann F, Acharya M (2008) The influence of lactose pseudopolymorphic form on salbutamol sulfate-lactose interactions in DPI formulations. Drug Dev Ind Pharm 34(9): 992-1001. 9. Das SC, Larson I, Morton DA, Stewart PJ (2010) Determination of the polar and total surface energy distributions of particulates by inverse gas chromatography. Langmuir 27(2): 521-523. 10. Van Oss C, Good R, Chaudhury M (1988) Additive and nonadditive surface tension components and the interpretation of contact angles. Langmuir 4(4): 884-891. 11. Van Oss CJ, Chaudhury MK, Good RJ (1988) Interfacial Lifshitz-van der Waals and polar interactions in macroscopic systems. Chem Rev 88(6): 927-941.