srep03968-s1
advertisement

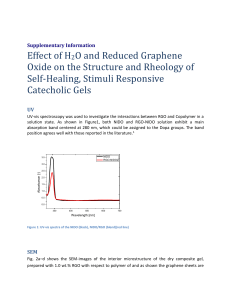
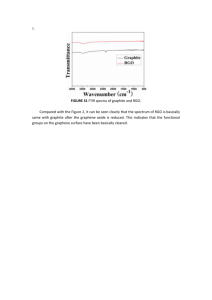
Supplementary Information Nano Conductive Ceramic Wedged Graphene Composites as Highly Efficient Metal Supports for Oxygen Reduction Peng Wu, Haifeng Lv, Tao Peng, Daping He and Shichun Mu* State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan 430070, China Fax: +86 27 87879468. E-mail: msc@whut.edu.cn Scheme S1. Formation of the Pt/RGO-ZrB2 and Pt/RGO catalysts 1 Figure S1. TEM image of RGO (a), RGO/ZrB2 (c) and SEM image of RGO (b), RGO/ZrB2 (d). 2 Figure S2. (a) ORR polarization curves of Pt/RGO-ZrB2 obtained at different rotating rates, (b) Koutecky-Levich plots of the current density reciprocal vs.ω-1/2 at different potentials. The ORR activities were further investigated using the Koutecky-Levich equations[1,2]: 1 1 1 1 1 1/ 2 J JL JK B JK (1) B 0.62nFC 0( D0) 2 / 3 1/ 6 (2) where J denotes the measured current density, JK is the kinetic current density, JL is the diffusion-limited current density, ω is the electrode rotation rate, F is the Faraday constant (96485 C mol-1), C0 is the bulk concentration of O2 (1.26×10-3mol L-1), D0 is the diffusion coefficient of O2 (1.93×10-5 cm2 s-1) and ʋ is the kinetic viscosity of the electrolyte (1.0×10-2 cm2 s-1). The electron transfer number (n) for Pt/RGO-ZrB2 is derived from the slope. 3 Figure S3 CV curves of the Pt/RGO/ZrB2 (a), Pt/RGO (b) and Pt/C (c) before and after ADT. 4 Figure S4. TEM image of the Pt/RGO (a,b), Pt/RGO-ZrB2 (c,d) and Pt/C (e,f ) catalysts after ADT 5 Figure S5. Nitrogen adsorption-desorption isotherms of ZrB2 and RGO-ZrB2 (hand milling). Figure S6. Nyquist plots for XC-72, RGO, RGO/ZrB2, ZrB2 (a); Pt/C, Pt/RGO, Pt/RGO-ZrB2 (b) in 0.5 M H2SO4. Inset is the equivalent circuit. 6 The EIS plots of all samples contain a partially overlapped semicircle. The charge transfer resistance in different supports can be estimated from the analysis of EIS spectra by using the software ZsimpWin based on an equivalent electrical circuit as shown in the inset of Figure S6.b, where Rel represents the uncompensated solution resistance, Rct is charge transfer resistance of the supporter and Qdl is a constant phase element [3,4]. Reference 1. Y. Hu, X. Zhao, Y. Huang, Q. Li, N. J. Bjerrum, C. Liu and W. Xing, J. Power Sources, 2013, 225, 129-136. 2. F. Jaouen, M. Lef`evre, J. P. Dodelet and M. Cai, J. Phys. Chem. B, 2006, 110, 5553–5558. 3. M. D. Stoller, S. Park, Y. Zhu, J. An and R. S. Ruoff, Nano Lett, 2008, 8, 3498. 4. S. Chen, J. W. Zhu and X. Wang, J. Phys. Chem. C, 2008, 112, 19841. 7