Leanne Blake`s Poster
advertisement

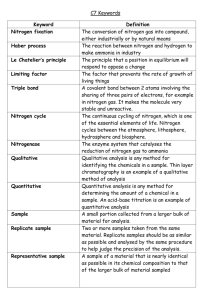
Abstract Analysis within chemistry comes in two formats; these include qualitative (aimed towards indicating the quality of the sample and therefore the purity) and quantitative analysis (used to identify how much of a compound is within a molecule for example). Using qualitative and quantitative analysis, it was possible for me to create an outreach workbook named ‘An introduction to analytical Chemistry’, using the methods below. Chromatography Many different practical techniques can be used within analytical chemistry; examples include chromatography, which can be used to simply identify different colours in a specific ink, but also to indicate the levels of different compounds within liquids and gases. Spectrophotometry The ability to measure a compound / compounds is an essential skill required in many areas of science. The most widely used method of doing so is with a technique called UV/visible Spectrophotometry. This method is based upon the fact that light interacts with molecules in a way which means that the material within the molecule can be identified. The spectra produced to show the results from gas and liquid chromatography can be used to identify unknown compounds, which makes the method useful within forensic sciences. Methods of separation like chromatography are becoming increasingly more popular and can even be used in drugs trials within the Olympics. Spectrophotometers contain a light source which emits white light; this then gets split into all of the contributing colours. Individual colours are directed towards the sample which can then absorb or transmit light. Light detectors then calculate the light which was both absorbed and transmitted, identifying the compound. Titrations Titrations are a form of quantitative analysis and help to calculate how much of one substance is needed to react with a given amount of another substance. The vitamin C titration, carried out within the workbook, is a redox titration between as vitamin C and Iodine. Starch is used in the titration as an indicator. The titration is carried out to find out the content of vitamin C within Vitamin C supplements. Data can then be used to compare and contrast the different levels of Vitamin C within the two different supplements. Flame Photometry Flame photometry (also known as flame atomic emission Spectrophotometry) is widely used to accurately measure the concentrations of alkaline metal ions (such as Na⁺ and K⁺) within water samples and samples of biological fluids. Flame photometers can therefore be used to detect the concentrations of pure metals. Due to this, flame photometry is a method of quantitative analysis. The main principal for flame photometry, is the fact that the compounds which make up the alkaline metals can be thermally dissociated. When this occurs, some of the atoms produced by the thermal dissociation can step up to higher level of energy. Once the atoms involved return once more to ground state, they emit radiation; some of this is visible as light. Each alkaline metal produces radiation at different wavelengths therefore the colours produced vary. Repeating titrations makes for better results, as this can determine whether or not results are accurate and fluent, this often means that the first run of a titration is usually used as a rough guide for when the titration will be complete. Conclusions Each of the methods mentioned above contribute towards analytical chemistry and are therefore presented in the final outcome of the outreach booklet. Methods used in analytical chemistry like chromatography and titrations often need to be repeated to determine correct results and collaboration.