Einstein`s Explanation of the Photoelectric Effect - BHS Physics
advertisement

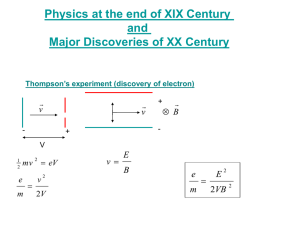
STAGE 2 PHYSICS READING Electricity and Magnetism Key Ideas pg 73-89 pg 101-104 Photoelectric Effect Key Ideas Intended Student Learning Photons Using graphical and algebraic methods, solve problems that require the use of Kmax hf W . Describe how microscopic observations of the building up of an image produced by light of very low intensity demonstrate the arrival of localised bundles of energy and momentum called ‘photons’. Calculate the energy and momentum of the photons in various regions of the electromagnetic spectrum. Describe how two-slit interference patterns build up over time when light of very low intensity is used. Photoelectric Effect When light of sufficiently high frequency is incident on matter, it may be absorbed by the matter, from which electrons are then emitted. This is called the ‘photoelectric effect’. Describe an experimental method for investigating the relation between the maximum kinetic energy of the emitted electrons (calculated from the measured stopping voltage) and the frequency of the light incident on a metal surface. The intensity of the incident light affects the number, but not the energy, of emitted electrons. Describe how Einstein used the concept of photons and the conservation of energy to explain the photoelectric effect. The minimum frequency f o at which electrons are emitted varies with the type of material and is called the ‘threshold frequency’. Deduce the equation Kmax hf W , where K max is the maximum kinetic energy of the emitted electrons. The work function W of a surface is the minimum energy required to remove an electron from it. Plot experimental values of maximum kinetic energy versus frequency, and relate the slope and horizontal and vertical intercepts to the equation Kmax hf W . The work function W is related to the threshold frequency by W hf o . Using graphical and algebraic methods, solve problems that require the use of Kmax hf W . Photons The particle model of light states that when light interacts with matter, it can behave as if it were made up of a stream of particles known as photons. This model can explain many properties of light that cannot be explained by the wave model, such as the photoelectric effect and production of xrays Photons are localised bundles (quanta) of energy and momentum. Individual photons have a frequency and wavelength; they do not however have any mass. Because of this, the general equations that apply to particles do not apply to photons Equations E= p= v= h= Questions 1. Light of 525nm is made up of photons find: a. The frequency of the light b. The momentum of the photons c. The energy of the photons Particle or Wave? The particle property of light was first demonstrated by conducting Young’s double slit experiment using low intensity light and a sensitive photographic film. Classical physics predicted very dim, but fully formed bands on the film. What was observed however looked like randomly scattered individual dots of light. Each dot corresponds to a single photon reaching the film. If left for an extended period of time a complete two slit interference pattern is produced. The Photoelectric Effect The photoelectric effect is the instantaneous emission of electrons when light strikes the surface of a metal. The light used must have a frequency above the threshold frequency for that particular metal. Electrons are bound to a metal by a particular amount of energy characteristic to that metal. To produce the photoelectric effect, the incident light must therefore have enough energy to free electrons from the metal. The threshold frequency is the lowest frequency such that the incident light has enough energy to cause electrons to be released. The work function is the smallest amount of energy binding electrons to the metal. It is given the symbol W. Therefore, for a particular metal, W = h.f0, where f0 is the threshold frequency. Experimental Method • The potential difference between anode and cathode is initially zero • When monochromatic light is shone on the metal cathode, electrons may be emitted. Some of these will travel across the gap to the anode. Thus, a current is produced in the circuit. • The potential difference is increased, which slows the electrons down as they cross the gap. The work done on these electrons is given by W = ΔV.q. • Therefore only those electrons with kinetic energy greater than this will make it across the gap • The potential energy is increased until the current decreases to zero. • Just before this, the only electrons that are able to cross the gap are those with the maximum possible kinetic energy • The maximum kinetic energy can then be calculated: • 𝐾𝑚𝑎𝑥 = 𝑊 = ∆𝑉. 𝑞 • ∴ 𝐾𝑚𝑎𝑥 = ∆𝑉. 𝑞 • These steps are repeated using different light sources and/or different metals. The results for each metal are then plotted on a graph of maximum energy versus frequency of light Experimental Observations 1. The maximum kinetic energy of the emitted electrons depends on the frequency of light and the metal used. The greater the frequency of light, the greater the maximum kinetic energy of the electrons. The relationship between maximum kinetic energy, frequency of light and the metal is 𝐾𝑚𝑎𝑥 = ℎ. 𝑓 − 𝑊 2. There is a minimum frequency of light that will eject photoelectrons (f0 for each metal used) 3. Increasing the intensity of light increases the magnitude of the current produced. However it does not affect the stopping voltage. This is because although the intensity of light increases the number of electrons emitted (due to more photons), it does not affect the maximum kinetic energy (as frequency remains the same) 4. Electrons are emitted the instant that light is shone on the metal Einstein’s Explanation of the Photoelectric Effect In order to explain the photoelectric effect Einstein developed the following concepts. A single photon passes its energy to a single electron A photon will transfer all or none of its energy An electron can only absorb the energy of one photon at a time Einstein used these concepts to explain the following properties of the photoelectric effect: Electrons are emitted the instant that light is shone on the metal. When a photon collides with an electron, it instantly passes its energy to the electron The greater the intensity of light, the more electrons are emitted The greater the intensity of light, the more photons will strike the metal. This means that more electrons will be emitted (as each photon can cause one electron to be emitted). The kinetic energy of the electron does not depend on the intensity of light. The intensity of the light determines the number of photons, but not the energy of the photons (which depends on the frequency). Each electron will only absorb the energy of one photon. Thus, an increased intensity will result in more electrons being emitted, but each electron will have the same energy as before. Emitted electrons may have a range of kinetic energies. Different electrons within a metal will be bound by different amounts of energy (greater or equal to W). The greater this amount of energy, the more energy is required to release the electron. A photon must have energy at least equal to W in order to release the least bound electron. If the photon has more energy than W it will be able to release electrons that are more strongly bound. Any energy greater than the binding energy will be converted into the kinetic energy of the emitted electron.