19 - Cabrillo College
advertisement
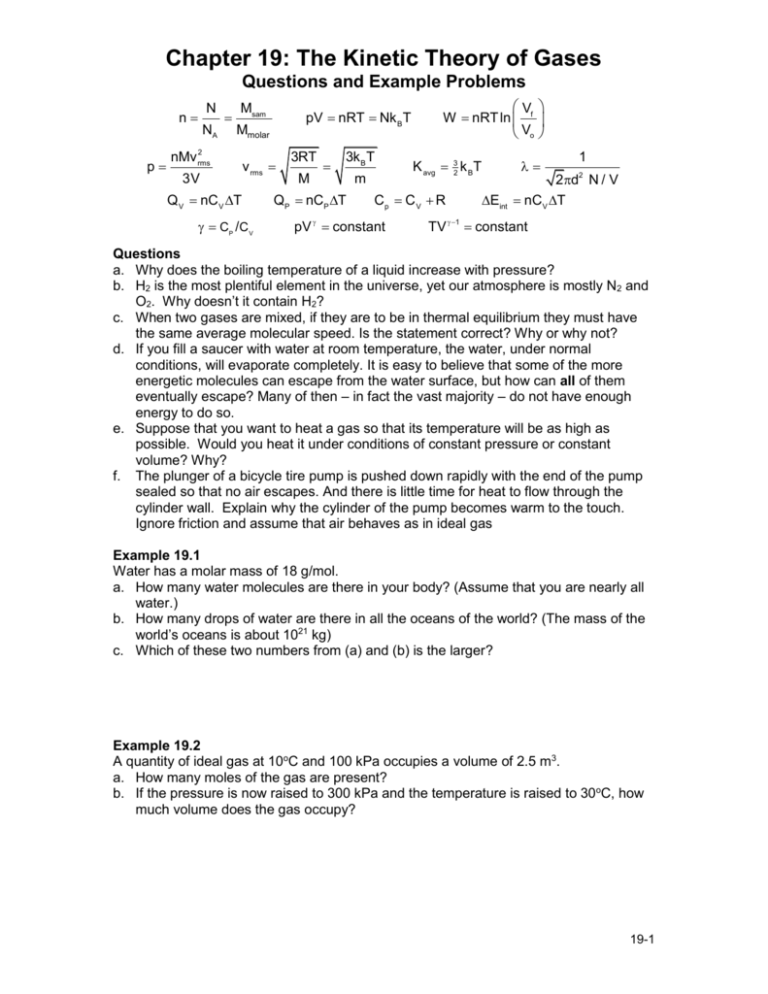
Chapter 19: The Kinetic Theory of Gases Questions and Example Problems N n p NA Msam pV nRT Nk BT Mmolar 2 nMv rms 3V v rms Q V nC V T CP / C V 3RT M 3k B T QP nCP T m Vf Vo W nRT ln K avg 32 k BT Cp C V R pV constant 1 2d N / V Eint nC V T 2 TV 1 constant Questions a. Why does the boiling temperature of a liquid increase with pressure? b. H2 is the most plentiful element in the universe, yet our atmosphere is mostly N2 and O2. Why doesn’t it contain H2? c. When two gases are mixed, if they are to be in thermal equilibrium they must have the same average molecular speed. Is the statement correct? Why or why not? d. If you fill a saucer with water at room temperature, the water, under normal conditions, will evaporate completely. It is easy to believe that some of the more energetic molecules can escape from the water surface, but how can all of them eventually escape? Many of then – in fact the vast majority – do not have enough energy to do so. e. Suppose that you want to heat a gas so that its temperature will be as high as possible. Would you heat it under conditions of constant pressure or constant volume? Why? f. The plunger of a bicycle tire pump is pushed down rapidly with the end of the pump sealed so that no air escapes. And there is little time for heat to flow through the cylinder wall. Explain why the cylinder of the pump becomes warm to the touch. Ignore friction and assume that air behaves as in ideal gas Example 19.1 Water has a molar mass of 18 g/mol. a. How many water molecules are there in your body? (Assume that you are nearly all water.) b. How many drops of water are there in all the oceans of the world? (The mass of the world’s oceans is about 1021 kg) c. Which of these two numbers from (a) and (b) is the larger? Example 19.2 A quantity of ideal gas at 10oC and 100 kPa occupies a volume of 2.5 m3. a. How many moles of the gas are present? b. If the pressure is now raised to 300 kPa and the temperature is raised to 30oC, how much volume does the gas occupy? 19-1 Example 19.3 A sample of ideal gas expands from an initial pressure and volume of 32 atm and 1.0 L to a final volume of 2.0 L. The initial temperature of the gas is 300 K. What are the final pressure and temperature of the gas and how much work is done by the gas during the expansion, if the expansion is isothermal? Example 19.4 Determine the average value of the translational KE of the molecules of an ideal gas at (a) 20oC (room temperature). (b) What are the rms values for (b) H2 and N2 at room temperature (20oC), (c) H2 in outer space, and (d) free electrons in the sun atmosphere (2×106 K)? Example 19.5 One mole of an ideal diatomic gas goes from 1 to 3 along two different paths as shown. a. Rank the (i) change in internal energy and (ii) heat transfers for transitions 1→3 and 1→2→3. Explain your reasoning. b. During the transition along 1→3, what is the (iii) change in internal energy of the gas and (iv) heating done on the gas? c. Repeat part (b) for transition 1→2→3. Example 19.6 The figure shows a thermodynamics process followed by 120 mg of helium. a. Determine the pressure, temperature, and volume of the gas at points 1, 2, and 3. Put your results in a table for easy reading. b. How much work is done on the gas during each of the three segments? c. How much heat energy is transferred to or from the gas during each of the three segments? Solution I need to figure out the signs and draw energy bar diagrams. a. Helium gas is a monatomic gas and the given number of moles is 19-2 mHe 120 10 3 g 0.030 mol n MHe 4 g/mol The underlying structure is the 1st law but the details are given by the IGL. In other words, to get the (p, V, T) at anyone state, is use the IGL equations with the IGL process to setup the equations. Let’s setup a table and fill-in the values to make sure I get them all. State-1: nRT1 p1V1 nRT1 p1 mol V1 nT0.030 1 133 273 406K 3 Transition 1→2 is V1 10 cm3 103 m3 n 0.030 8.31 406 Pa 1.01 105 Pa 1 atm 103 isochoric (constant volume process) so that the IGL implies T T p V constant 1 2 T2 2 T1 5T1 2030 K p1 p2 p1 T1 406K p2 /p1 5 Transition 2→3 is isothermal so T constant p2 V2 p3 V3 V3 p2 V1 5V1 5 103 m3 p1 T1 406K p2 /p1 5 I now summarize everything into my table p(105Pa) T(K) V(m3) 1 1 406 10-3 2 5 2030 10-3 3 1 2030 5×10-3 b. To determine the work done through each transition, we do the following: Transition 1→2 is isochoric: W 12 = 0 Transition 2→3 is isothermic: V 5 W23 p2 V2 ln 3 5 105 Pa 103 m3 ln 815 J W23 1 V2 Transition 2→3 is isobaric: W31 p3 (V1 V3 ) 1.01 105 (1 5) 103 405 J W31 c. To determine the heating through each transition, we do the following: Transition 1→2 is isochoric heating: Q12 Eint nCV T 32 nR(T2 T1 ) 32 (p2 V2 p1V1 ) 3 2 5 10 5 103 1 105 103 Pa m3 609 J Q12 Transition 2→3 is isothermic heating: Q23 W23 815 J Q23 Transition 2→3 is isobaric heating: Q31 nCP T 52 nR(T1 T3 ) 32 (p1V1 p3 V3 ) 5 2 1 105 103 5 105 103 J 1013 J Q31 19-3 Example 19.7 In a bottle of champagne, the pocket of gas (primarily carbon dioxide) between the liquid and the cork is at pressure of p1 = 5.00 atm and temperature of 5oC. When the cork is pulled from the bottle, the gas undergoes an adiabatic expansion until its pressure matches the ambient air pressure of 1.00 atm. a. Draw a pV-diagram for the situation and what is the ratio of the molar specific heats? Explain. b. What is its temperature at the end of the adiabatic expansion? Solution Let p1, V1, and T1 represent the pressure, volume, and temperature of the initial state of the gas, and let p2, V2, and T2 be the pressure, volume, and temperature of the final state. Since the process is adiabatic p1V1 = p2V2. Combining with the ideal gas law, pV = nRT, we obtain p1V1 p1(T1 / p1 ) p11 T1 constant p11 T1 p12 T2 With = 4/3 which gives (1−)/ =−1/4, the temperature at the end of the adiabatic expansion is p T2 1 p2 1 5.00 atm T1 1.00 atm 1/4 (278 K) 186 K 87C T2 . 19-4 Example A The temperature of 3.00 mol of a gas with CV = 6.00 cal/mol∙K is to be raised 50.0 K. a. If the process is at constant volume, draw a pV-diagram of the situation and determine Q, W, ∆Eint, and ∆K (total translational kinetic energy) of the gas? b. Repeat (a) if instead the process is at constant pressure. c. Repeat (a) if instead the process is adiabatic. Solution I would expect that because the internal energy and the translation kinetic energy are temperature dependent, these values will be the same for all three processes. Furthermore, since the question specifically states translational kinetic energy, it means monatomic: CV =3R/2. The given quantities are T = 50 K, n = 3.0 mol, and converting joules to calories in the ideal gas constant value gives R ≈ 2.0 cal/mol·K. a. Constant volume process: since the work done by the gas is W = 0, and the change in the internal energy is Eint nCV T 3.0 6.0 50 900 cal Eint The first law gives Eint Q W Q 900 cal The change in the total translational kinetic energy is K 32 nRT 32 3.0 2.0 50 450cal K b. Constant pressure process: since the change in internal energy is the same as above (∆Eint = 900 cal), the work done at constant pressure is W pV pV nRT nRT 3.0 2.0 50 300 cal W The first law gives Q Eint W 900 300 1200cal Q The change in the translational kinetic energy is K 32 nRT 32 3.0 2.0 50 450cal K c. Adiabiatic process: once again the change in internal energy is the same as above (∆Eint = 900 cal) but by definition of adiabatic Q =0. The first law leads to Eint Q W W 900 cal The change in the translational kinetic energy is K 32 nRT 32 3.0 2.0 50 450cal K Example B An ideal gas undergoes an adiabatic compression from (p, V, T) = (1.0 atm, 1.0×106 L, 0.0oC) to (p, V) = (1.0×105 atm, 1.0×103 L). (a) Is the gas monatomic, diatomic, or polyatomic? (b) What is its final temperature? (c) How many moles of gas are present? (d) What is the total translational kinetic energy per mole before and after the compression? Solution a. We use p1V1 = p2V2 to compute : ln V V ln 1.0 10 ln p1 p2 2 1 5 monatomic L 3 ln 1.0atm 1.0 105 atm 3 L 1.0 106 b. Using the gas law in ratio form, the final temperature is 19-5 T2 T1 p2 V2 p1V1 1.0 10 273K 5 atm 1.0 10 3 L 1.0 atm 1.0 10 6 L 2.7 10 4 K c. The number of moles of gas present is 1.01 105 Pa 1.0 103 cm3 p1V1 n 4.5 104 mol RT1 8.31 J/mol K 273K d. The total translational energy per mole before the compression is K1 32 RT1 32 8.31 J/mol K 273K 3.4 103 J and after the compression, K 2 32 RT2 32 8.31 J/mol K 2.7 10 4 K 3.4 10 5 J 19-6











