Platyhelminthes *Part 1
advertisement

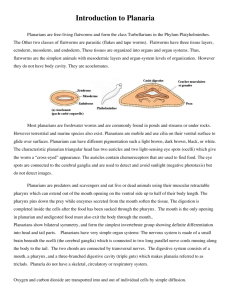
Platyhelminthes –Part 1-Freeliving forms. Background information Flatworms, or Platyhelminthes, are bilaterally symmetrical metazoans with three tissue layers. Unlike most triploblastic animals, they are compact and have no coelom (body cavity) surrounding the viscera and no hemal system. The gut, if present, has a single opening to the exterior. Osmoregulation and fluid regulation is accomplished with protonephridia. The end product of nitrogen metabolism is ammonia, which is lost by diffusion across the body surface. Gas exchange is by diffusion, which is facilitated by small body size and flattening. Nutrients are delivered to tissues by diffusion from the gut, whereas oxygen diffuses across the body surface. An anterior brain with associated concentration of sense organs is present, as expected of bilaterians. Light-sensitive pigment-cup ocelli occur in various positions on the body. Flatworms are complex animals with elaborate hermaphroditic reproductive systems. Fertilization is internal with copulation. Most are hermaphroditic. They may be free-living or parasitic. Dugesia tigrina is one of several species of freshwater triclad flatworms collectively known as "planarians". Activities: Four groups will conduct behavioral experiments while the rest of the class examines internal and external anatomy. The groups running the behavioral experiments need to be respectful of classmates and inform them when they are done, so the others have time to conduct the behavioral studies. 1. Behavioral studies---Chemotaxis: Use brown or black freshwater planaria. We need to keep cultures of freshwater and marine planarians separate, so make sure that you do not mix these and return your specimens to the proper jar. ). Living worms can be picked up with a plastic pipette but they must be ejected quickly or they will attach to its wall using their adhesive glands. They adhere tenaciously and are difficult to remove when this happens. We will use a training T maize or a habitat maze to investigate chemotaxis in planaria. This is the first group to exhibit fairly complex sensory structures. In the laboratory, you will have available liver and egg yolk and some commercial preparations. You can do a competitive experiment or simply place one of the stimuli at the end of one of trails and see if the planarians given a choice can detect the stimulus, presumably following the chemical gradient caused by food dissolving in water and find either the egg yolk or liver. If you are fortunate, you will see the worm protrude its pharynx and feed. The sight is impressive and the length of the pharynx will may startle you. You may also want to investigate what other classes have dubbed the “social influence”. They have placed two to three planarians in one well, none in the other and found that other planarians are particularly “good” at finding other planarians. Do your results substantiate their findings? Do the other planarians have to be feeding or just hanging out? Get approval of your experimental plan before proceeding. If the class wishes they can try to “train” some planarians to always take one fork. One group can determine their preferences and then turn their fast learners over to another group to see if they can train them. Some specimens of planarians are surprisingly fast learners, refusing after a few trials to try the other fork, even after the food has been removed and both should be providing no stimulus. Alive in the literature (1998 article) is still the suggestion that feeding such “smart” or trained worms to others results in them learning faster. It is claimed that when trained planaria are sliced in half and allowed to regenerate, both the head- and tail-derived worms demonstrate significant recall of their original training. They are the choice of training models in some college psychology classes. They habituate to puffs of air and beams of light (disliking intensely both stimuli). This is also the way they now are “trained” to go to the left or right in training maize. 2. Living Specimens: anatomy and locomotion Use light brown, white freshwater planaria or horse shoe crab flatworms (marine) if available. If both are available each pair of students at a table should work with different species. You will get better feeding videos with the horseshoe crab flatworms, but better locomotion and HIGH magnification shots with the freshwater planarians. Your notebook should contain a comparison (accompanied by photographs) of the marine and freshwater species if available. Place a living worm in a small pool of water on a small petri dish (Restrict movement by restricting the water available or you will not be able to follow your worms). Living worms can be picked up with a plastic pipette but they must be ejected quickly or they will attach to its wall using their adhesive glands. They adhere tenaciously and are difficult to remove when this happens. a. Observe the worm with the dissecting microscope. Watch it as it moves across the slide. Time the movement and divide the distanced moved by body length. The major locomotory force is produced by the cilia of the ventral epidermis but muscular activity also plays a role in locomotion, especially in making turning movements. Manipulate the worm with a tiny needle to encourage it to change directions. Try to discover the contributions of the musculature to this maneuver and think about which muscles would be involved in making a turn. Freshwater specimens provided are poor, ineffective swimmers and often just coil up and drop until they find a substrate upon which they can move. b. Make a movie of an unstained planarian moving. In your journal, describe planarian movement. c. You should try to feed your worms. In light colored specimens this will make the digestive tract obvious. Horse shoe crab flatworms will feed on cut up black worms. Freshwater planaria will prefer fresh beefheart, liver or fish food flakes. Photograph or make a movie of your planarian feeding. d. Push the worm if it stops gently with the needle and look for evidence of adhesive ability. Where do the adhesive cells seem to be located? e. Observe the animal with transmitted light and look for the intestine and its cecae after it is feed. You will have to add water to the dish to observe feeding. Take of a photograph of an animal that has recently fed and label its digestive tract. If the animal does not feed, try photographing the cilia. Transmitted light against a black background may also enable you to see cilia better. HIGH MAGNIFICATION: d. Working in pairs place a freshwater planarian on a slide and position a cover slip over the worm. Place the slide on the compound or inverted microscope. The weight of the cover slip will squeeze the worm thereby immobilizing it and making it thin enough to see some internal structure. Do not use 400X on these slides. Focus, with 100X, on the edge of the head, reduce the light, and look for evidence of beating cilia. Most of the animal's cilia are ventral and thus difficult to see in a whole mount but the head bears cilia associated with chemosensory receptors on the auricles and their activity is obvious. The name "turbellaria" means "little disturbance" and is a reference to the movement of water caused by the cilia of the auricles. Increase the light so you can illuminate some of the interior. At 200X you may be able to see gut diverticula, especially if the animal has eaten recently. The pharynx is the conspicuous, long, pale area in the center of the body. If the animal is squeezed sufficiently, you may see the pharynx clearly and you may even see the opening at its posterior end. The pharynx will probably move about in the pharyngeal chamber and may increase in length. Use the diagram below to help you identify important structures. In most specimens you will be able to see parts of the digestive tract, eyespots and pharynx. Other structures will not generally be visible but they are included in the diagram so you can see their relationship to the visible structures. You can also find a more detailed diagram and description under exercise two which asks you to look cross section of a planarian and a whole mount if you cannot discern structures in your living specimens. Slides Whole mount Slides: If you were able to obtain a good photograph of a living specimen, or one in which you could clearly identify pharynx, eye spots and digestive tract, (or obtained a striking photo of one of these at high power) you do not have to examine the whole mount slide. Every pair needs to examine a slide containing a cross-section of a planarian. a. With the dissecting microscope or low power of the compound microscope study a whole mount of a fixed and stained planarian. Note the dorsoventral flattening and bilateral symmetry. Locate the anterior-posterior axis, which is the axis of symmetry. The plane of symmetry includes this axis and divides the worm into right and left sides. Specimens are usually mounted on the slide with the back, or dorsal surface, up. Try to identify as many features as you can in the labeled diagram of a whole mount. Before the end of lab, you should gather as a group and discuss the features found. If you obtained a very good picture of the living specimen, you do not need to photograph the whole mount. You can use the description below to guide you through the examination. The anterior end of the body is the head and the remainder is the trunk. Dugesia tigrina has a pointed triangular head but the head of some species is blunt and rounded. The posterior limit of the head is marked by a pair of lateral, ciliated, chemosensory protrusions, the auricles. These are variously developed in different species and are sometimes absent. The two dark ocelli, or eyespots, are easily seen near the middle of the head. The digestive system is best studied using a specimen that has been fed a colored substance such as carmine powder or carbon black and thus has a gut filled with pigment. A whole mount of such a specimen should be available, either on the slide you are now using or mounted separately. Locate the cylindrical pharynx lying on the midline at the center of the body. The pharynx is a muscular, protrusible tube housed in a spacious pharyngeal cavity, which opens to the exterior via the mouth. The pharyngeal lumen, which may contain pigment, occupies the center of the pharynx. The mouth, which is rarely visible on whole mounts, is a small pore on the ventral midline of the body immediately posterior to the pharynx. It is the opening of the pharyngeal cavity to the exterior. During feeding, the pharynx lengthens to many times its resting length and is extended out of the mouth to reach the food. The proximal end of the pharynx opens into the intestine, which, in triclads, immediately divides into three branches one anterior and two posterior rami. The name Tricladida (clad = branch) alludes to the presence of the three rami. Each ramus ends blindly and bears abundant ceca, or diverticula, so that no area of the body is beyond effective diffusion distance from the food source. Figure 1. Labeled whole mount of a typical planarian. Numerous protonephridia are present but are not visible in these preparations. Sometimes some features of the reproductive system can be seen posterior to the pharynx but it is rare that enough can be discerned to make sense of it. Cross-section Slides a. You main goal is to obtain a photograph of a labeled section that identifies the various muscles. Do not try to identify the different types, just indicate their presence (one side is sufficient) with arrows. Indicate on the slide however where organs of the three embryonic layers can be found. Examine a prepared slide of a planaria cross sections. Most commercial slides have three cross sections taken at different levels along the worm. There is usually one through the anterior end of the body, one through the pharynx and one through the posterior part of the worm. Slides are not uniform however, and do not necessarily conform to this ideal, nor can you rely on the order in which the sections are arranged on the slide. There are diagrams of a typical pharyngeal and non-pharyngeal cross section to help you identify muscles. This is the first “official” group of triploblastic animals and so you will need to keep in mind the relationship between the three tissue layers and the organs observed. Locate and identify each of the three sections on your slide. Be sure you know dorsal from ventral. The ventral surface is usually flatter than the arched dorsal surface. The pharyngeal section is usually easy to recognize. “A good pharyngeal section shows the large, unmistakable pharynx as a hollow, red circle surrounded by a narrow white ring in the center of the section. The anterior and posterior sections have one to many irregular circles distributed through the cytoplasm. These circles are intestinal rami and ceca. Most of the body proper consists of tissue dedicated to obtaining or utilizing the nutrients obtained.” The descriptions below each diagram are offered to help you find important structures and again not to be memorized. Relationship of tissue layers to structures observed. Detail of a typical (not a pharyngeal section) section. Figure b. Posterior cross section through a planarian The body is covered by a monolayered, secretory epidermis (Fig 2). The ventral, but not the dorsal, epidermis is ciliated, a fact that can be verified by careful observation with high power (400X). The cilia are used for locomotion and the cells are multiciliated. The dorsal surface is not ciliated. Compare the dorsal and ventral epithelia to be sure you can recognize cilia. The epidermis is underlain by a distinct basal lamina, which is visible as a thin, dark line just inside the epidermis. The dorsal epidermis contains numerous secretory vesicles and rod-shaped secretions, the rhabdites (rhabd = rod). Rhabdites are synthesized by epidermal gland cells submerged below the basal lamina into the parenchyma. When expelled at the surface, rhabdites become sticky mucus, which may help trap small invertebrate prey. Note the clusters of adhesive gland cells situated at the lateral edge of the ventral epidermis. These are part of a ciliafree adhesive zone that encircles the worm. These cells secrete an adhesive that helps the animal grip the substratum. The ventral epidermis bears numerous gland cells that secrete mucus. A thick layer of body wall muscles, consisting of outer circular and inner longitudinal fibers, lies just inside the epidermis. Inside the muscle layer, the interior of the worm is filled with a mesenchymal connective tissue, the parenchyma, consisting of cells in a fibrous extracellular matrix and having a loose, open appearance. Many large epidermal gland cells are submerged into it but they nevertheless open to the surface through the epidermis. Dorsoventral muscles can be seen passing vertically through the parenchyma connecting the muscle layers of the dorsal and ventral body walls. These muscles maintain the flat shape of the triclad body. Scattered about in the interior are sections through the intestinal rami and their ceca. These are irregular circles of various sizes and unpredictable number. The clear space in the interior of each is the gut lumen and is surrounded by a monolayered epithelium of large, vacuolated cells, which may be secretory, absorptive, or phagocytic. This epithelium is usually referred to as the gastrodermis, or mucosal epithelium. c. Pharyngeal Cross-section Figure c. Cross section through the pharyngeal region of a planarian. The pharynx occupies almost the entire center of the section and the body wall is very thin above and below it. Locate and identify the two white, unstained spaces associated with the pharynx. The one in the center of the pharynx is the pharyngeal lumen whereas that surrounding the pharynx is the pharyngeal cavity. This type of pharynx, known as a plicate pharynx and consisting of a muscular tube retractable into a sheath is characteristic of triclads and polyclads. Moving outward from the lumen in the center of the pharynx, the layers are, in order: pharynx lumen, gastrodermis, inner muscle, parenchyma (connective tissue), outer muscle, epithelium, pharyngeal cavity, and epithelium of the pharynx sheath (Fig 3). 3. REGENERATION OF PLANARIA: Use only brown freshwater planaria Freahwater planatians typically reproduce asexually by a type of fission in which the worm divides into two fragments without prior differentiation of new parts. Transverse cleavage just posterior to the pharynx divides the worm into an anterior, nearly normal, worm with head, mouth, pharynx and most of the gut, and an incomplete, headless posterior mass of tissues, which must replace its missing parts. Following division, the anterior end behaves normally but the posterior end remains immobile until regeneration is complete and the missing parts replaced. You are not likely to see fission occurring but, if your laboratory maintains populations in aquaria, it is quite possible that you will see these headless lumps stuck on the walls of the aquaria. In the experiment you have done, if you cut the worm into two pieces at least, you were initiating a natural process, although probably not at the scheduled desired by your worm In spite of natural process, scientists were amazed to learn that a planarian often divided into 5 pieces or more, still retains the ability to regenerate a whole animal from most of the segments. The regenerative abilities depend on neoblasts which are scattered throughout the planatian body. Neoblasts apparently remain in an unspecialized, stem cell state, which enables them to differentiate into any cell type. Wherever planaria are cut, the neoblasts migrate to the site and form a blastema by themselves. As classic as this experiment appears, geneticists are again turning to planaria to provide answers about development despite the fact that neoblasts are unique to this phylum. The new approach is to slip genes into the neoblasts or mutate existing genes, and then add the cells to worms whose own neoblasts have been destroyed with radiation. When such worms regenerate, any new cells should derive from the genetically engineered neoblasts. The object is to look at which genes are active in these organisms during regeneration and even if the cells involved are different, similar genes may be involved in wound healing or development in some way in other organisms. a. Your task as a class is to design an experiment that will test whether gradients occur in regenerative abilities. Note that the gradient can be head to tail, the reverse or actually initiated from mid body with regeneration decreasing in efficiency as one moves from mid body to head or tail. Some pairs need to divide the animal into threes. The class may wish to discuss what other experimental manipulations will tell them about the gradient of regeneration. Procedure: Prepare a divided Petri dish to receive your segments. Mark each section with a different letter. As one partner places segments in the dish, another should keep tract of which section of the dish, a particular segment of the dissected worm is placed. Using a large bore pipette or butterfly forceps, remove a Planarian from the culture and place it in the Petri dish and than on a damp piece of waxed paper. Once it stretches out, place the waxed paper with the Planarian on an ice cube or dish containing shaved ice. Make sure that you have several layers of waxed paper between the planarian and the ice. Your goal is to use the ice to immobilize the planarian without freezing the animal. Frozen or “dead” tissue does not regenerate. Never keep your specimen on ice for very long. You goal should be to keep the animal on ice only long enough to cut it into segments (one or two minutes at the most). With a scalpel or razor blade, quickly cut the Planarian. Place each segment in a separate section of the petri dish. We will keep the Petri dishes in a dark place at room temperature. Each table should try to prepare three dishes. We will try to examine the Planaria for a few minutes every two or three days and produce a record of regeneration. Next week you will examine your specimens and discuss your results. For next week: As a class analyze your results. Each person write their own report Analysis: After examining the results of the Planaria regeneration experiments, did you observe any pattern in the way that the regeneration occurred? If so, describe the pattern. Does it indicate any gradient in regeneration efficiency? If you had to predict where neoblasts are most found, where would that be and why? Many of the sections of the Planaria will likely die before regeneration is complete. Why might this have happened? If a group would like to pursue any experiments with planaria as their project, we can accommodate this for regeneration and chemotaxis experiments. Both of these activities involve considerable outside of class lab time to complete these projects.