Platyhelminth
advertisement

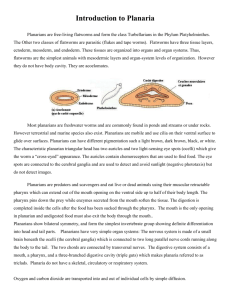
Platyhelminthes –Part 1-Freeliving forms. Background information Flatworms, or Platyhelminthes, are bilaterally symmetrical metazoans with three tissue layers. Unlike most triploblastic animals, they are compact and have no coelom (body cavity) surrounding the viscera and no hemal system. The gut, if present, has a single opening to the exterior. Osmoregulation and fluid regulation is accomplished with protonephridia. The end product of nitrogen metabolism is ammonia, which is lost by diffusion across the body surface. Gas exchange is by diffusion, which is facilitated by small body size and flattening. Nutrients are delivered to tissues by diffusion from the gut, whereas oxygen diffuses across the body surface. An anterior brain with associated concentration of sense organs is present, as expected of bilaterians. Light-sensitive pigment-cup ocelli occur in various positions on the body. Flatworms are complex animals with elaborate hermaphroditic reproductive systems. Fertilization is internal with copulation. Most are hermaphroditic. They may be free-living or parasitic. Dugesia tigrina is one of several species of freshwater triclad flatworms collectively known as "planarians". Activities: Four groups will conduct behavioral experiments while the rest of the class examines internal and external anatomy. The groups running the behavioral experiments need to be respectful of classmates and inform them when they are done, so the others have time to conduct the behavioral studies. 1. Behavioral studies---Chemotaxis: Use brown or black freshwater planaria. Leave light colored specimens for microscopic examination of structures. Living worms can be picked up with a plastic pipette but they must be ejected quickly or they will attach to its wall using their adhesive glands. They adhere tenaciously and are difficult to remove when this happens. We will use a training T maize or a habitat maze to investigate chemotaxis in planaria. This is the first group to exhibit fairly complex sensory structures. In the laboratory, you will have available liver and egg yolk and some commercial preparations. You can do a competitive experiment or simply place one of the stimuli at the end of one of trails and see if the planarians given a choice can detect the stimulus, presumably following the chemical gradient caused by food dissolving in water and find either the egg yolk or liver. If you are fortunate, you will see the worm protrude its pharynx and feed. The sight is impressive and the length of the pharynx will may startle you. You may also want to investigate what other classes have dubbed the “social influence”. They have placed two to three planarians in one well, none in the other and found that other planarians are particularly “good” at finding other planarians. Do your results substantiate their findings? Do the other planarians have to be feeding or just hanging out? Get approval of your experimental plan before proceeding. If the class wishes they can try to “train” some planarians to always take one fork. One group can determine their preferences and then turn their fast learners over to another group to see if they can train them. Some specimens of planarians are surprisingly fast learners, refusing after a few trials to try the other fork, even after the food has been removed and both should be providing no stimulus. Alive in the literature (1998 article) is still the suggestion that feeding such “smart” or trained worms to others results in them learning faster. It is claimed that when trained planaria are sliced in half and allowed to regenerate, both the head- and tailderived worms demonstrate significant recall of their original training. They are the choice of training models in some college psychology classes. They habituate to puffs of air and beams of light (disliking intensely both stimuli). This is also the way they now are “trained” to go to the left or right in training maize. 2. Living Specimens: anatomy and locomotion. Use very light brown, or white freshwater planaria if available. If both are available each pair of students at a table should work with a different species. Place a living worm in a small pool of water on a small petri dish (Restrict movement by restricting the water available or you will not be able to follow your worms). Living worms can be picked up with a plastic pipette but they must be ejected quickly or they will attach to its wall using their adhesive glands. They adhere tenaciously and are difficult to remove when this happens. a. Observe the worm with the dissecting microscope. Watch it as it moves across the slide. Time the movement and divide the distanced moved by body length. The major locomotory force is produced by the cilia of the ventral epidermis but muscular activity also plays a role in locomotion, especially in making turning movements. Manipulate the worm with a tiny needle to encourage it to change directions. Try to discover the contributions of the musculature to this maneuver and think about which muscles would be involved in making a turn. Freshwater specimens provided are poor, ineffective swimmers and often just coil up and drop until they find a substrate upon which they can move. Make a movie of a planarian moving. In your journal, describe planarian movement. b. Push the worm if it stops gently with the needle and look for evidence of adhesive ability. Answer these questions in your journal. Where do the adhesive cells seem to be located? How do you know? c. You should try to feed your worms. In light colored specimens this will make the digestive tract obvious. Freshwater planaria will prefer fresh beefheart, liver or fish food flakes. Photograph or make a movie of your planarian feeding. d. Observe the animal with transmitted light and look for the intestine and its cecae after it is feed. You will have to add water to the dish to observe feeding. Take of a photograph of an animal that has recently fed and label its digestive tract. If the animal does not feed, try photographing the cilia. Transmitted light against a black background may also enable you to see cilia better. e. Your notebook should also contain a comparison (accompanied by photographs) of the marine and freshwater species if available. Our marine species when available is a small white flatworm that lives on horseshoe crabs. The Limulus flatworm (Bdelloura) is found around the book gills and leg joints of crabs, especially on older females that have not shed for a long time How is it's body modified for life attached to another organism.? Bdelloura candida (Tricladida), ectocommensal on the king crab, Limulus. Complete digestive and male systems are shown on the bottom half of the animal, female systems on top half. _________________________________________________________________________ HIGH MAGNIFICATION (If you can manipulate the worm enough under the stereoscope to see its pharynx, cecae, eyespots and cilia, you do not have to the high magnification observations.) Use however the diagrams in this section to help you label planarian structures. Working in pairs place a freshwater planarian on a slide and position a cover slip over the worm. Place the slide on the compound or inverted microscope. The weight of the cover slip will squeeze the worm thereby immobilizing it and making it thin enough to see some internal structure. Do not use 400X on these slides. Focus, with 100X, on the edge of the head, reduce the light, and look for evidence of beating cilia. Most of the animal's cilia are ventral and thus difficult to see in a whole mount but the head bears cilia associated with chemosensory receptors on the auricles and their activity is obvious. The name "turbellaria" means "little disturbance" and is a reference to the movement of water caused by the cilia of the auricles. Increase the light so you can illuminate some of the interior. At 100X you may be able to see gut diverticula, especially if the animal has eaten recently. The pharynx is the conspicuous, long, pale area in the center of the body. If the animal is squeezed sufficiently, you may see the pharynx clearly and you may even see the opening at its posterior end. The pharynx will probably move about in the pharyngeal chamber and may increase in length. Use the diagram below to help you identify important structures. In most specimens you will be able to see parts of the digestive tract, eyespots and pharynx. Other structures will not generally be visible but they are included in the diagram so you can see their relationship to the visible structure. Label photographs accordingly, be truthful and label only those structures you can see. __________________________________________________________________________ 3. Do together as a class. REGENERATION OF PLANARIA: Use only brown or black freshwater planaria Freahwater planatians typically reproduce asexually by a type of fission in which the worm divides into two fragments without prior differentiation of new parts. Transverse cleavage just posterior to the pharynx divides the worm into an anterior, nearly normal, worm with head, mouth, pharynx and most of the gut, and an incomplete, headless posterior mass of tissues, which must replace its missing parts. Following division, the anterior end behaves normally but the posterior end remains immobile until regeneration is complete and the missing parts replaced. You are not likely to see fission occurring but, if your laboratory maintains populations in aquaria, it is quite possible that you will see these headless lumps stuck on the walls of the aquaria. In the experiment you have done, if you cut the worm into two pieces at least, you were initiating a natural process, although probably not at the scheduled desired by your worm In spite of natural process, scientists were amazed to learn that a planarian often divided into 5 pieces or more, still retains the ability to regenerate a whole animal from most of the segments. The regenerative abilities depend on neoblasts which are scattered throughout the planatian body. Neoblasts apparently remain in an unspecialized, stem cell state, which enables them to differentiate into any cell type. Wherever planaria are cut, the neoblasts migrate to the site and form a blastema by themselves. As classic as this experiment appears, geneticists are again turning to planaria to provide answers about development despite the fact that neoblasts are unique to this phylum. The new approach is to slip genes into the neoblasts or mutate existing genes, and then add the cells to worms whose own neoblasts have been destroyed with radiation. When such worms regenerate, any new cells should derive from the genetically engineered neoblasts. The object is to look at which genes are active in these organisms during regeneration and even if the cells involved are different, similar genes may be involved in wound healing or development in some way in other organisms. a. Your task as a class is to design an experiment that will test whether gradients occur in regenerative abilities. Note that the gradient can be head to tail, the reverse or actually initiated from mid body with regeneration decreasing in efficiency as one moves from mid body to head or tail. Some pairs need to divide the animal into threes. The class may wish to discuss what other experimental manipulations will tell them about the gradient of regeneration. Procedure: Prepare a divided Petri dish to receive your segments. Mark each section with a different letter. As one partner places segments in the dish, another should keep tract of which section of the dish, a particular segment of the dissected worm is placed. Using a large bore pipette or butterfly forceps, remove a Planarian from the culture and place it in the Petri dish and than on a damp piece of waxed paper. Once it stretches out, place the waxed paper with the Planarian on an ice cube or dish containing shaved ice. Make sure that you have several layers of waxed paper between the planarian and the ice. Your goal is to use the ice to immobilize the planarian without freezing the animal. Frozen or “dead” tissue does not regenerate. Never keep your specimen on ice for very long. You goal should be to keep the animal on ice only long enough to cut it into segments (one or two minutes at the most). With a scalpel or razor blade, quickly cut the Planarian. Place each segment in a separate section of the petri dish. We will keep the Petri dishes in a dark place at room temperature. Each table should try to prepare three dishes. We will try to examine the Planaria for a few minutes every two or three days and produce a record of regeneration. Next week you will examine your specimens and discuss your results. For next week: As a class analyze your results. Each person writes their own report Analysis: After examining the results of the Planaria regeneration experiments, did you observe any pattern in the way that the regeneration occurred? If so, describe the pattern. Does it indicate any gradient in regeneration efficiency? If you had to predict where neoblasts are most found, where would that be and why? Many of the sections of the Planaria will likely die before regeneration is complete. Why might this have happened? 4. Acoel anatomy The acoels are small ciliated worms without a lumen to the gut. The epidermis is a simple epithelium bearing the cilia and is underlaid by a grid-work of muscles. The cilia provide gliding and swimming movement, and the muscles, both those of the body wall and deeper-lying ones crossing through the body, steer the body with turning and twisting motions as well as pull the body wall in movements that grab food and stuff it through the mouth. The digestive tissue consists of a syncytium within which vacuoles form around food that is ingested. The nervous system is largely in the form of a nerve net, but a centralized mass surrounding the statocyst constitutes a brain from which stronger nerves extend as longitudinal cords.The statocyst with a single highly refractile statolith is located anteriorly and can often be seen in the dissecting microscope. In many species there are prominent glands that open through an anteroterminal pore; these are the frontal glands. In mature animals, eggs are easily detected as very large cells with a large nucleus. Discerning the testes usually requires higher magnification. They are normally located anterolaterally to the ovaries, but there are exceptions. There are many different types of male copulatory organs including a simple pore, a ciliated antrum, a muscular penis or a sclerotized stylet. A female gonopore is present in most, but not all species. Female accessory organs may be present in the form of a bursa for storage of allosperm. In some species the bursa is equipped with a bursal nozzle, a narrow passage through which allospermatozoa have to pass in order to fertilize the oocytes. Other features of importance for identification of acoels are pigment patterns, presence of a pharynx (in a small number of species), presence of symbiotic algae, and body shape and size. Record locomotion in your specimen. Can you find the statocyst or see the cells of the digestive tract through the pigmentation? a. Below are diagrams to help you locate important structures. Label these structures on photographs of acoels. Obviously depending on the size of the “worm” and color you may or may not see a structure. Be honest in your journal but do label those structures you can see in acoels. Below Parts of the digestive system=================Cilia/muscle tracks Nervous system=============Male reproductive system====Female reproductive system Ventral side showing position of mouth in some species b. In your journal, compare the structure of an acoel with that of the tubellarians or more complex flatworms. Confine your comments to structures you can see.