chemistry_unit_2_revision - pisscience
advertisement

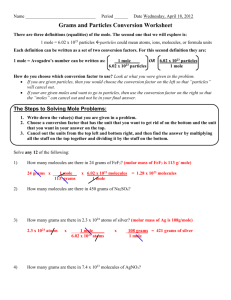
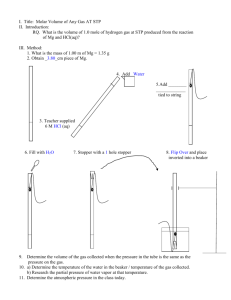
Port Said International Schools Better Education for Future Generations Science Department Grade: 10 Unit 2 Revision Name: ………………………………………. Date: …………………………………………………… Write the scientific term: 1. Expresses the chemical symbols and formulas of the reactants and products. .……………………………………………………………………………………… 2. The smallest part of a substance that can be found in a single state. The properties of the substance are evident in it. .……………………………………………………………………………………… 3. The smallest building unit of the substance that participated in chemical reactions. ……………………………………………………………………………………… 4. The mass of one atom. .…………………………………………………………………………………… 5. The mass of one molecule in grams. .……………………………………………………………………………………… 6. The sum of atomic mass of the atoms forming that molecule. .……………………………………………………………………………………… 7. The amount of one of the reactants when it is less than its molar ratio in the balanced chemical equation. .……………………………………………………………………………………… 8. The number of atoms, molecules or ions found in one mole of the substance and equals 6.02 ✕ 1023 (atom, molecule or ion.) .……………………………………………………………………………………… 9. The amount of matter that contains Avogadro’s number of molecules. .……………………………………………………………………………………… 10.The volume of gas involved in the reaction and produced from it both are in a fixed ratios. .……………………………………………………………………………………… 11.The equal volumes of different gases contain the same number of molecules under the same standard temperature and pressure. ……………………………………………………………………………………… 12.The number of units from the particle for each 100 units from the overall. .……………………………………………………………………………………… 13.A formula expressing the simplest ratio of true numbers between the atoms of elements which formed the compound. .……………………………………………………………………………………… 14.A symbolic formula of the molecule of the element, or molecule or the formula unit. It expresses the actual type and number of atoms or ions that form this molecule or unit. .……………………………………………………………………………………… http://pisscience.wikispaces.com/ Port Said International Schools Better Education for Future Generations Science Department Grade: 10 Unit 2 Revision 15.The amount of a substance which we practically obtain from a reaction ..………………………………………………………………………………… 16.The amount of the calculated substance based on the reaction's equation. .……………………………………………………………………………………… Give reason for the following: 1. The volume occupied by 26 g of acetylene C2H2 at (STP) is equal to the volume occupied by 2 g of hydrogen at the same conditions. ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… …………………………………………...………………………………………………… 2. The difference in the molar mass of phosphorus by the difference in its physical state. ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… …………………………………...………………………………………………………… 3. One liter of oxygen gas contains the same number of molecules that a liter of chlorine gas contains at STP. ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………….……………………………………………………… 4. The number of molecules of 9 g of water (H2O) is equal to the number of molecules of 39 g aromatic benzene C6H6. ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… 5. The chemical equation should be balanced. …………………………………………………………………………………………….. ……………………………………………………………………………………………… http://pisscience.wikispaces.com/ Port Said International Schools Better Education for Future Generations Science Department Grade: 10 Unit 2 Revision 6. When you calculate the gas volume in terms of its molar mass, it should be placed in the standard conditions of pressure and temperature. ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………… 7. The practical yield is always less than the theoretical yield of the equation. ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ………………………………………………………………...…………………………… 8. The molar mass of the sulfur in the solid state differs from its molar mass in the gaseous state. ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ………………………………………………………………………………………………. Choose the correct answer: 1. Number of water moles found in 36 g of it are (0.5 – 1 – 2 – 2.5) moles. 2. Number of carbon dioxide molecules found in 128 g of it equals (2 – 6.022 × 1023 – 3.011× 1023 – 12.044 × 1023) molecules. 3. Number of produced sodium ions from dissolving 40 g of NaOH in water equals (2 – 6.022 × 1023 – 3.011× 1023 – 18 × 1023) ions. 4. The volume of 4 g of hydrogen molecules H2 in standard conditions (STP) equals (2 – 22.4 – 44.8 – 89.6) liter. 5. The volumes of producing gasses from the reaction are directly proportional with the participating gas volumes in the reaction (Avogadro’s Law - Avogadro’s number Jay-Lussac Law - Law of Mass Reservation). 6. The number of the empirical formula units of the compound C2H2O4 is (1 – 2 – 3 – 4). 7. The mass of CaO resulted from dissociating 50 g of calcium carbonate CaCO3 thermally is (28 – 82 – 96 – 14) grams. 8. The number of hydrogen moles needed to produce 11.2 L of water vapor at (STP) is (22.4 – 44.8 – 0.5 – 68.2) liter. 9. If the empirical formula of a compound is CH2 and its molecular mass is 56 , then the molecular formula of such a compound is (C3H6 - C2H4 – C4H8 – C5H10). 10. The masses of atomic particles are estimated by the unit of atomic masses (a.m.u) which equals (one atomic mass unit equals ????? in grams) (6.022 × 1023 – 1.66 × 1023 – 1.66 × 10-23 – 1.66 × 10-24) grams. Calculate in Kg. 11.The unit used in the international system SI to express the amount of a http://pisscience.wikispaces.com/ Port Said International Schools Better Education for Future Generations Science Department Grade: 10 Unit 2 Revision substance is (gram – kilogram – mole – unit of atomic masses). 12.The number of grams of 44.8 L of ammonia gas NH3 at (STP) equals (2 – 17 – 34 – 0.5) grams. 13.If an amount of sodium contains 3.01 ✕ 1023 atoms, then the mass of this amount equals (11.5 – 23 – 46 – 0.5) grams. 14.A chemical equation is to be balanced to satisfy (Avogadro - Reservation of energy Reservation of mass - Gay $ Lussac). 15.Half a mole of carbon dioxide CO2 is (44 – 22 – 88 – 66). 16.The empirical formula of CH2O expresses the molecular formula of (HCHO CH3COOH - C6H12O6). 17.When 64 g of oxygen reacts with abundance of hydrogen, then the volume of the water vapor resulted in STP is (22.4 - 44.8 - 11.2 - 89.6). Solve the following problems: First: Calculate the molecular formula for a compound contains carbon with a ratio 85.7 % and hydrogen with a ratio 14.3 % and its molecular mass is 42. ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………...…………………… Second:130 g of silver chloride precipitated when a mole of sodium chloride dissolved in water reacted with silver nitrate solution. Calculate the following: .1 The percentage of the actual product. ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………..…………………………………………………… .2 The number of sodium ions resulted from this reaction. ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… .……………………………………………………………… http://pisscience.wikispaces.com/ Port Said International Schools Better Education for Future Generations Science Department Grade: 10 Unit 2 Revision Third: Calculate the number of moles of 144 g of carbon. ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… …………………………………….………………………………………………………… Fourth: Calculate the mass of 2.4 mole of a limestone CaCO3 ……………………………………………………………………………………………… ……………………………………………………………………………………………… ......................………………………………………………………………………………… ................................................................................................................................................ Sixth: Calculate the volume of 56 g of nitrogen at (STP). ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ………………………………………………………………..…………………………… Seventh: Calculate the volume of hydrogen and the number of sodium ions resulted from the reaction of 23 g of sodium and an excess amount of water in the standard conditions according to the following equation: ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ………………………………………………………..…………………………………… Eighth: Calculate the volume of one mole of phosphorus in the gaseous state at (STP), and then calculate the number of the atoms in this volume. ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ………………………………………………………..…………………………………… http://pisscience.wikispaces.com/ Port Said International Schools Better Education for Future Generations Science Department Grade: 10 Unit 2 Revision Ninth: 39.4 g of barium sulfate BaSO4 precipitated when 40 g of barium chloride solution BaCl2 reacted with an abundance of potassium sulfate. Calculate the percentage of the practical yield of barium sulphate. ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………… Tenth: Calculate the ratio of iron present in the raw siderite FeCO3. ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… ……………………………………………………………………………………………… http://pisscience.wikispaces.com/ Port Said International Schools Better Education for Future Generations Science Department http://pisscience.wikispaces.com/ Grade: 10 Unit 2 Revision