Supplementary Material Effect of concentrated polymer brushes on
advertisement

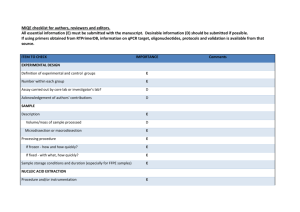
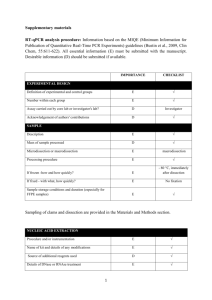
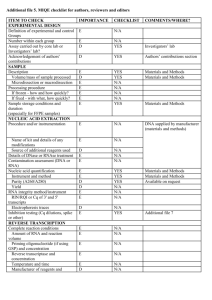
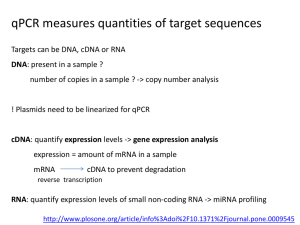
Supplementary Material Effect of concentrated polymer brushes on the expression of inflammatory and angiogeneic genes in human umbilical vein endothelial cells Chiaki Yoshikawa,1* Yoshihisa Shimizu,1 Junji Suzuki,1 Jun Qiu,2 Edith van den Bosch3* 1World Premier International Research Center for Materials Nanoarchitectonics, National Institute for Materials Science (NIMS), Tsukuba, Ibaraki 305-0047, Japan 2DSM Ahead, P.O. Box 18, 6160 MD Geleen, The Netherlands 3DSM Biomedical, P.O. Box 18, 6160 MD Geleen, The Netherlands Materials 2-Hydroxyethyl acrylate (HEMA) (99%, Wako Pure Chemical, Osaka, Japan) was purified as previously reported [1]. Poly(ethylene glycol) methyl ether mehtacrylate (average Mn ~475) (PEGMA) (Aldrich) was purified by passing through neutral alumina. Cu(I)Br (99.9%, Wako Pure Chemical, Japan), ethyl 2-bromoisobutylate (EBIB) (99%, Wako), 2, 2’-bipyridine (bpy) (99%, NacalaiTesque, Japan), methanol (99%, Nacalai), anisol (99%, Wako), Lipopolysaccharide (LPS) from E. coli O127 Phenol extraction (Wako) were used as received. (2-bromo-2isobutyloxy)propyltriethoxysilane (MUBIB) was synthesized according to the literature [2]. Preparation of the patterned CPB surfaces. A patterned gold silicon wafers were immersed in an ethanol solution of MUBIB (4 wt%) for 48 h at room temperature and washed several times with ethanol. Using the patterned substrate, CPBs of PPEGMA and PHEMA were prepared by SI-ATRP as our previous report [1, 3]. As an example, SI-ATRP of PPEGMA was shown as follows: The MUBIB immobilized substrates were immersed in an Ar purged methanol solution of PPEGMA (0.96 M), Cu(I)Br (4.8 mM), bipyrydine (9.6 mM), and the free initiator EBIB (4.8 mM) and the solution was kept at 30 oC for 3h. After the polymerization, the solution was diluted with eluent and analyzed by the GPC. The PPEGMA grafted substrates were also washed with methanol. HUVEC culture. Human umbilical vein endothelial cells (HUVEC) (Lonza, Basel, Switzerland) were cultured in endothelial basal medium 2 (Lonza) supplemented with EGM-2 SingleQuots (Lonza) (2% FBS) at 37 oC in a humidified atmosphere of air containing 5% CO2. At subconfluence, the cells were removed from the tissue culture plate polystyrene by trypsin treatment. The concentration of the HUVECs was adjusted to 3.0104 cells/cm2 and the cells were seeded on samples and cultured for 24 h at 37 oC in a humidified atmosphere of air containing 5 % CO2. For LPS treatment, cultured medium on substrate was exchanged LPS (1 µg/ml) containing medium after 22 h from start of cell culturing. The cells were cultured for additional 2 h. After culturing for 24 h, the substrates were rinsed three times with fresh PBS and stored at -80 ˚C until RNA extraction. Visualization of adhered cells. Following cell culture, the cells on the test substrates were washed three times with PBS and fixed with 4% paraformaldehyde in PBS for 20 min at room temperature. The samples were permeabilized with 0.1% Triton X-100 in PBS, and incubated with Phalloidin-Alexa546 (Invitrogen, MA, USA) to visualize F-actin. The stained cells were further washed and mounted with ProLong Gold antifade reagent with DAPI (Invitrogen). The adherent cells were observed by a fluorescence microscope. RNA isolation and first strand cDNA synthesis Total RNA was extracted from the HUVEC using NucleoSpin RNA II (MACHEREY-NAGEL, Düren, Germany), according to manufacturer’s protocol. The genomic DNA were removed with digestion by DNase on the column. The RNA contents were quantified by measuring the absorbance at 260 nm using a Nanodrop ND-1000 spectrophotometer (NanoDrop Technologies, Inc., Wilmington, DE). All RNA samples had a 260/280 ratio greater than 2.0. First strand cDNAs were synthesized from each 1 µg of total RNA, with using ReverTra Ace® qPCR RT Kit (TOYOBO, Japan) according to manufacturer’s protocol. Quantitative real-time PCR. Gene-specific primer sequences were selected from Primerbank (http://pga.mgh.harvard.edu/primerbank/) (Table S1). All primer pairs were analyzed by Blast to ensure specificity for the intended target gene within the human genome. Quantitative real-time PCR (qPCR) reactions were carried out in a LightCycler® ST300 (Roche, Basel, Switzerland) using the following thermal profile: 95 ˚C for 10 minutes, 45 cycles at 95 ˚C for 10 seconds, 55 ˚C for 20 seconds, and 72°C for 20 seconds. PCR amplification using a LightCycler instrument was performed in 15 μL of reaction mixture consisting of a master mixture containing FastStarttaq DNA polymerase, a dNTP mixture, buffer (LightCycler DNA Master SYBR Green I, Roche), 2.0 mM of MgCl2, 0.5 μM of each primer, and 15 ng of template DNA in a LightCycler Capillary. A dissociation curve was generated for each plate following the amplification cycles by slowly cooling from 95 oC at a 2% ramp rate. PCR reactions occurred in a final volume of 10 µL, containing 5 µL of SYBR GreenMaster Mix (Applied Biosystems), 1 µL of each 10 µM of forward and reverse primers, and 10 ng of cDNA template in 4 µl of RNase/DNase free water. The qPCR data analysis was performed as described in the literature [4]. Briefly, individual crossing point (Cp) values and mean amplicon efficiencies were estimated using LinRegPCR (Version 11.0, download: http://LinRegPCR.HFRC.nl). The relative quantity (RQ) of each template was normalized to the RQ of the endogenous control, GAPD or β-actin, for each sample to give the normalized relative quantity (NRQ) for every gene of interest (GOI). The relative expression ratio (R) was calculated as the ratio of the mean NRQ(treated) to the mean NRQ(control). Results were expressed as the mean relative expression ratio ± standard error (n = 3). Statistical analysis of differences in the number of successful PCR amplifications was performed by Student’s t-tests. Values with P < 0.05 were considered significant. References [1] Yoshikawa C, Goto A, Tsujii Y, Fukuda T, Yamamoto K, Kimura T, Kishida A. Macromolecules 2006;39:2284. [2] Jones D. M, Brown A. A, Huck W. T. S Langmuir 2002, 18: 1265. [3]Yoshikawa C, Hashimoto Y, Hattori S, Honda T, Zhang K, Terada D, Kishida A, Tsujii Y, Kobayashi H. Chem. Letters2010;39:142. [4] Rieu I, Powers S. J The Plant Cell, 2009, 21: 1031. Supplementary Table 1. Gene-specific primer sequences. Gene Sequence IL-6 for AATTCGGTACATCCTCGACGG IL-6 rev TTGGAAGGTTCAGGTTGTTTTCT VEGF for CGCAGCTACTGCCATCCAAT VEGF rev GTGAGGTTTGATCCGCATAATCT IL-8 for ACTGAGAGTGATTGAGAGTGGAC IL-8 rev AACCCTCTGCACCCAGTTTTC GAPD for AAGGTGAAGGTCGGAGTCAAC GAPD rev GGGGTCATTGATGGCAACAATA -actin for CATGTACGTTGCTATCCAGGC -actin rev CTCCTTAATGTCACGCACGAT Supplementary Scheme 1. Schematic illustration of the preparation of patterned CPBs. Supplementary Fig. 1. qPCR analysis of IL-6, IL-8 and VEGF expression of HUVECs cultured for 24h on micro-patterned PPEGMA-CPBs. The copy number of genes was normalized with -actin. Y-axis was normalized with the values obtained on silicon wafers. Values are mean ± SEM (n = 3). *P < 0.05. Supplementary Fig. 2. qPCR analysis of IL-6, IL-8 and VEGF expression of HUVECs cultured for 24h on micro-patterned PHEMA-CPBs. The copy number of genes was normalized with -actin. Y-axis was normalized with the values obtained on silicon wafers. Values are mean ± SEM (n = 3). *P < 0.05.