XPS: X-ray Photoelectron Spectroscopy Analysis & Applications
advertisement

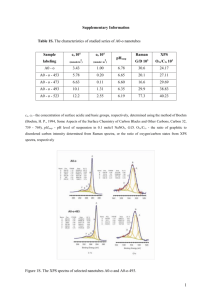
X-ray photoelectron spectroscopy Arena Holguin, Sylvia Natividad, Gabriel Gonzalez 1. Introduction X-ray photoelectron spectroscopy is a form of electron spectroscopy in which a sample is irradiated with a beam of monochromatic x-rays and the energies of the resulting photoelectron are measured. The physicist K. Siegbahn initiated the electron spectroscopy for chemical analysis (ESCA) now known as X-ray photoelectron spectroscopy (XPS) and its applications in science. In 1981 Siegbahn won the Novel Prize for his contribution to his work on XPS, which is a very helpful technique in obtaining information about oxidation state and atomic composition of analyzed compounds. XPS involves a single electron process which is based on the photoelectron effect described by Albert Einstein.1 2. XPS mechanism process X-ray Photoelectron Spectroscopy, XPS, uses either monochromatic Aluminum Kα or nonmonochromatic Magnesium Kα x-rays to eject a photoelectron from an atom at the sample’s surface. An electron from a higher energy level then falls to fill the hole left behind and its emitted radiation energy is used to eject an Auger electron. Thus, XPS emits both photoelectrons and Auger electrons which can be seen in the spectrum (wide peaks). The electrons ejected are analyzed in the XPS detector by measuring the electrons kinetic energy which provides the information to determine the kind of elements present in the sample. Figure 1 illustrates the schematic representation of the x-ray photoelectron process. Emitted Auger electron 1 Skoog, Holler and Nieman. Principles of Instrumental Analysis. Fifth Editon 538-539. Figure 1 Schematic representation of the X-ray photoelectron process2. The kinetic energy of the ejected electrons is named Ek, and it is determined by the following equation. Ek= hv-Eb- ϕ The binding energy of an electron is represented by Eb, the energy of a photon is given by hv where h represents the plank’s constant and v is the frequency of a photon. The work function is represented by the symbol ϕ which is the minimum energy needed to remove an electron from a solid. Figure 2 summarizes graphically the equation Ek= hv-Eb- ϕ.3 Figure 2 summarizes graphically the equation E k= hv-Eb- ϕ 4 3. XPS analytical properties The X-ray photoelectron spectroscopy detects the electron energies and identifies the elements and oxidation states of the atoms in a sample. The XPS spectrum shows a number of emitted electrons against their kinetic energy. The XPS method has very important applications on solid surfaces and as nondestructive method of analysis of compounds5. 2 http://webh01.ua.ac.be/mitac4/micro_xpsaes.pdf James W. Robinson, Eileen M. Skelly Frame, George M. Frame II. Undergraduate Instrumental Analysis. Sixth Edition 880-881. 4 http://www.mri.psu.edu/facilities/MCL/events/presentations/AugerXPSopenhouse06212007.pdf 5 Strobel Heineman. Chemical Instrumentation: A Systematic Approach. Third Edition 807-808. 3 The XPS method is based on core electron ejection analysis which provides a very important qualitative technique since the spectral interface are very small and the XPS peaks for the electrons have good resolution. The peaks on the XPS method have chemical shift which lead to the identification of oxidation states of elements and the chemical composition of the sample. The extraordinary resolution of XPS peak are due to monochromatic Al K-alpha X-rays and the XPS mechanism of a single electron transition from the core electrons. 4. XPS analytical paramenters It is important to understand the XPS parameters such as the maximum depth so at the time of the sample analysis we can obtain good peaks and results. For instance the maximum depth range for alloys and pure metal is 2.5 nm and for organic compounds the maximum depth range is 10 nm. As part of the XPS instrument it has a cylindrical mirror analyzer which measures a spectrometry range of 100-1500eV. Another important parameter of the XPS instrument is the ultra high vacuum system that in order for the XPS to operate effectively it should run at 1x10-9 Torr vacuum pressure.6 5. XPS applications in research and industry Example 1 XPS has the capability to provide information about the surface layer in thin film structures which are important in research and industrial applications. Such applications include corrosion, adhesion, polymer surface, semiconductor and catalysis. For example, figure 3 shows an impurity in the polymer surface that shows the presence of fluorine within it.7 Figure 3 A contaminated polymer surface that shows the presence of fluorine in the contaminated area. 6 7 Dr Jorge Lopez Lectures and notes. www.phi.com Example 2 The diagram on figure 4 shows an actual XPS spectrum obtained from a Pd metal sample using Mg Ka radiation the main peaks occur at kinetic energies of about 330, 690, 720, 910 and 920 eV. ϕ Figure 4 Real XPS spectrum obtained from a Pd metal sample using Mg Ka radiation the main peaks occur at kinetic energies of ca. 330, 690, 720, 910 and 920 eV8 Example 3: Surface characterization and catalytic activity of sulfate, molybdate and tungstate promoted Al2O3–ZrO2 solid acid catalyst X ray photoelectron spectroscopy was used to characterize ultra-thin oxide surfaces (1-10nm). The photoelectron peaks of O 1s, Zr 3d, and Al 2p are shown on figures 5-7 respectively9. Figure 5. The Zr d XPS spectra of Al 2O3–ZrO2 and promoted Al2O3–ZrO2 samples calcined at 650 ◦C: (AZ) Al2O3–ZrO2; (SAZ) SO4 2−/Al2O3–ZrO2; (MAZ) Mo/Al2O3–ZrO2; (WAZ) W/Al2O3-ZrO2. 8 http://mmrc.caltech.edu Benjaram M. Reddy, Pavani M. Sreekanta, Yusuke Yamada, Tetsuhiko Kobayashi . Surface characterization and catalytic activity of sulfate-, molybdate- and tungstate-promoted Al2O3–ZrO2 solid acid catalyst. Journal of Molecular Catalysis A: Chemical 227 (2005) 81–89. 9 Figure 6. The O 1s XPS spectra of Al2O3–ZrO2 and promoted Al2O3–ZrO2 samples calcined at 650 ◦C: (AZ) Al2O3– ZrO2; (SAZ) SO4 2−/Al2O3–ZrO2; (MAZ) Mo/Al2O3–ZrO2; (WAZ) W/Al2O3 Figure 7. The Al 2p XPS spectra of Al2O3–ZrO2 and promoted Al2O3–ZrO2 samples calcined at 650 ◦C: (AZ) Al2O3–ZrO2; (SAZ) SO4 2−/Al2O3–ZrO2; (MAZ) Mo/Al2O3–ZrO2; (WAZ) W/Al2O3 7. Synopsis X-ray photoelectron spectroscopy (XPS) is a method that can be applied to the analysis of surface chemistry on a sample of interest. XPS analyzes the electronic state, chemical state, and chemical composition of elements in a sample. It also analyzes the kinetic energy of electrons that are ejected from the atoms in the sample when tit is irradiated with a beam of x-rays which determines the elements present in the sample. Finally XPS can be used to analyze the uniformity of elemental composition, chemical state, and electronic state of elements in the surface.10 10 http://en.wikipedia.org/wiki/X-ray_photoelectron_spectroscopy