Recipient Preop Lab Draws
advertisement

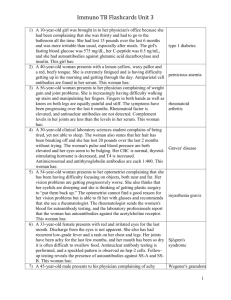
Policy: Kidney/Pancreas Pre-Transplant: Lab Draw Protocol Statement: : Activation date: 2/27/06 2. Affected Department: Kidney/Pancreas Transplant Program 3. Vision Strategy: Patient Care 4. Policy Statement: The Emory Transplant Center will comply with all applicable federal, state and local laws, regulations and policies regarding the management patient’s laboratory draws and results. 5. Basis: This policy is necessary for the protection of patients, physicians and staff 6. Administrative Responsibility: Section heads, physicians, practitioners, and staff are responsible for compliance with this policy. Scope/Procedure: 7. Protocol: a. All patients will have laboratory testing done with each visit to the Outpatient Transplant Center. *NOTE: 2nd Group & Type (ABORH) will only be performed at Day 2 of the patient’s transplant evaluation. b. Different appointment types will require specific laboratory testing as noted below. c. Physician may add or remove additional testing as appropriate for individual patient. Day One of the Evaluation Standard Orders ABO RH Type (need Blood Bank Request) CBC (WBC, RBC, HGB, HCT, PLT) does not include differential Differential, Automated CMV Total (IgG & IgM) Comprehensive Metabolic Hepatitis A Antibody (IgG & IgM) Hepatitis B (diagnostic profile) Hepatitis C Antibody HIV Antibody Lipid (total cholesterol, HDL, LDL, triglycerides) Phosphorous Protime (PT/INR) PTT (partial thromboplastin) RPR (rapid plasma regain) Toxicology Drug Screen, Blood Uric Acid Varicella Zoster Virus Antibody Urine Tests Toxicology Drug Screen, Urine (for patients who are not currently on dialysis) Urinalysis, Routine Urine Culture & Sensitivity Miscellaneous Amylase HCG Blood (qualitative) (women <50) CEA (history of colon cancer) C-Peptide (type I diabetics) Hepatitis B DNA by PCR Hemoglobin A1C (all diabetics) PTH Protein Electrophoresis (SPEP) PSA Screen (Medicare only on all men 40 years and older) Sickle Cell Screen (African American recipients) T4 (free) TSH (thyroid stimulating hormone) Fabry’s (is to be ordered on all recipient evaluation and re-evaluation patients EXCEPT the following: PCKD, established etiology of renal failure (biopsy confirming no-Fabry’s cause i.e., lupus nephritis, fsgs, etc.), congenital or urological etiologies, type I diabetics (K/P) or other double organ transplant candidates (heart/kidney, liver/kidney) Mis cell ane ous Urin e 24 hour urin e colle ctio n for total prot ein, total crea tinin e and crea tinin e clea ranc e HLA Orders HLA Antibody Screen Lupus Orders – On all patients with a history of Lupus Anti-DNA Histone Antibody Anti-Nuclear Antibody C3 Complement C4 Complement Lupus Anticoagulant Profile Phospholipids Hypercoagulation Orders Anti-cardiolipin Antibody Anti-thrombin 3 Factor 5 Leiden Protein C Protein S HIV Orders – On all patients with a history of HIV CD3/CD4/CD8 Quantitative EBV Panel G6PD HIV Quant./RT-PCR/Ultra sensitive Lactic Acid Blood Toxoplasma IgG Anemia Orders Vitamin B12 (protect from light) Ferritin (serum) Folate Iron & TIBC Reticulocyte Count Day 2 of the Evaluation Group and Type (ABORH)ABC (Class I) Molecular Typing DR/DQ (Class II) Molecular Typing Autologous Crossmatch Class I Specificity Testing Class II Specificity Testing Re-Evaluation Lab Standard Orders CBC (WBC, RBC, HGB, HCT, PLT) does not include differential Differential, Automated Comprehensive Metabolic Hepatitis A Antibody (IgG & IgM) Hepatitis B (diagnostic profile) Hepatitis C Antibody HIV Antibody Lipid (total cholesterol, HDL, LDL, triglycerides) Phosphorous Protime (PT/INR) PTT (partial thromboplastin) RPR (rapid plasma regain) Toxicology Drug Screen, Blood Uric Acid Varicella Zoster Virus Antibody Urine Tests Toxicology Drug Screen, Urine (for patients who are not currently on dialysis) Urinalysis, Routine Urine Culture & Sensitivity Miscellaneous HCG Blood (qualitative) (women <50) Hemoglobin A1C (all diabetics) PSA Screen (Medicare only on all men 40 years and older) HLA Orders (As determined by HLA department) Class I Specificity Testing Class II Specificity Testing HLA Antibody Screen Approved by: Kidney/Pancreas Transplant Leadership Group _____________________________________ Tom Pearson, M.D. Chair, Kidney/Pancreas Transplant Leadership Group Director, Kidney/Pancreas Transplant Program Related Policies/Procedures: Pre-transplant Re-evaluation Procedure