Notebook Check Grade Sheet pages 132-158
advertisement

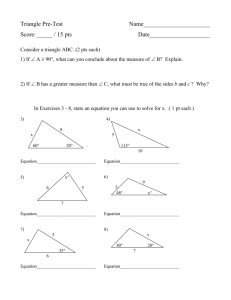
8th Grade Notebook Check Pages 132-159 Page 132- Bell Work 3/25: Answer the following questions in complete sentences: 1. The lower the pH, the more _____________ a substance is. 2. Why is acid rain bad for buildings and statues made of limestone? (CaCO3) "Physical and Chemical Changes" practice worksheet taped in and completed. Bell Work 3/26: Draw a Venn diagram comparing and contrasting physical and chemical changes. Page 133- "Physical and Chemical Changes" Cornell notes from reading, which should be taped in and marked. Page 134- Bell Work 3/27: Answer the following in complete sentences. 1. The mess in Love Canal has been cleaned up, and a new subdivision has been built. Would you ever consider living in the new neighborhood if you could get a house at half the cost? Why or Why not? 2. Calculate the density if mass= 250g and a volume= 50 mL. 3. Calculate the density if volume= 60mL and mass= 120g. Bell Work 3/28: Answer the following in complete sentences. 1. My cat is overweight, so I have to keep track of his weight to try to get him “back on track.” However, the only scale doesn’t detect his weight when I place him on the scale. Without buying a new scale, how can I figure out the weight of my cat? Page 135- "Love Canal Timeline" Text taped in and marked, completed timeline underneath. (10 items) Page 136- Bell Work 4/3: "Genpets.com Reflection" Browse site, then write a 4-5 sentence reflection. What do you think of this new product? Any comments, concerns, or questions? Page 137- "Reliability and Bias" notes (on handout received in class) Page 138-139: Bell Work 4/7: Create a list for the following question1. When I think of a “city,” I think of… Bell Work 4/8: Create a list for the following question2. What services should your city provide for its residents? What does a city need to function successfully and attract people to live there? Page 140- "The Lorax" video guide, completed. "Sustainability" defined. Bell Work 4/15: Respond to the following in complete sentences1. The Onceler’s way of manufacturing “Thneeds” was not sustainable- it damaged the environment AND was unable to continue for future generations. What could he have done instead to make it more sustainable? Bell Work 4/16: Respond to the following in complete sentences1. How do human activities contribute to greenhouse gases? +1 all BW’s labeled +1 BW 3/25 complete +3 BW 3/26 complete ___________ / 5 pts. ___________ / 5 pts. +1 all BW’s labeled +2 BW 3/27 complete +2 BW 3/28 complete ___________ / 5 pts. ___________ / 5 pts. ___________ / 5 pts. ___________ / 5 pts. +1 all BW’s labeled +2 BW 4/7 complete +2 BW 4/8 complete ___________ / 5 pts. +3 video guide complete +2 BW both complete ___________ / 5 pts. Page 141- "Surrounded by Pollution" article summary or Bell Work 4/17: Read the article “Surrounded by Pollution,” then respond to the following in complete sentences1. What are the causes of air pollution? 2. Why is the effect so severe now? 3. What are the effects of air pollution? +2 summary +3 questions answered in complete sentences ___________ / 5 pts. Page 142- Bell Work 4/21: Respond to the following in complete sentences1. How are plastics both a positive and negative man-made product? 2. What are some solutions to solve the problem of garbage patches in the oceans? Page 143- "Greenhouse Gases" notes (see ppt. above) ___________ / 5 pts. Page 144- "Alternative Resources" notes from cubes ___________ / 5 pts. Page 145- "Plastics" notes (see ppt. above) ___________ / 5 pts. Page 146-147: "Pollution Problems and Solutions" vocabulary defined (Closeup Alternative to Lorax Flyer) STEM essay vocab. defined. ___________ / 5 pts. Page 148- Bell Work 4/24: Respond the the following in complete sentences. 1. Why are we looking for alternative resources to fossil fuels? 2. Which alternative do you think is most likely to be successful? Why? Bell Work 4/28: Respond to the following in complete sentences1. Reflect on your life as a consumer- someone who is the final user of goods and services. Which of these good or services do you think are the worst for the environment? How do you think your impact on the environment compares to that of the rest of America? ***As you watch “The Human Footprint,” analyze how you compare to the “average American.” This should take up most of the page! Page 149- "The Penny Lab" taped in and completed. Bell Work 4/29- Respond to the following in complete sentences. 1. How does one product, like a diaper, create so much waste? "Human Footprint" Reflection (after viewing video) Bell Work 4/30- Respond to the following in complete sentences. 1. What are some simple things you can do to help the environment? ___________ / 5 pts. +1 BW 4/24 complete +1 BW 4/28 complete +3 Human Footprint reflection ___________ / 5 pts. +3 Penny Lab complete +1 BW 4/29 complete +1 BW 4/30 complete ___________ / 5 pts. Page 150- Bell Work 5/1: Respond to the following in complete sentences. 1. How do we know if a change is physical or chemical? Bell Work 5/2: How many atoms of each element are in the following molecules? (Be sure to write the name of each element, not just the symbol!) 1. KCl 2. KClO3 3. C2H3NaO2 Bell Work 5/5: How many atoms of each element are in the following molecules? (Be sure to write the name of each element, not just the symbol!) 1. Ag2O 2. Fe3O4 3. CH3CH2CH3 4. Al(OH)3 Bell Work 5/6: Determine the number of atoms of each element and the molecular mass. (Be sure to write the name of each element, not just the symbol!) 1. H3PO4 2. Ba(OH)2 3. Ag2O 4. Na2O2 Page 151- "Chemical Formulas" notes Page 152- "Counting Atoms" practice worksheet Bell Work 5/7: Find the differences between these two images: (in class). Bell Work 5/8: Determine the number of atoms of each element and the molecular mass. (Be sure to write the name of each element, not just the symbol!) 1. KMnO4 2. Na2SO4 Bell Work 5/9: Balance the following chemical equations: 1. NaHCO3 --> H2 + NaCO3 2. Pb(NO3)2 + KI --> PbI2 + KNO3 3. C6H12O6 + O2 --> CO2 + H2O +3 Penny Lab complete +1 BW 4/29 complete +1 BW 4/30 complete ___________ / 5 pts. ___________ / 5 pts. +1 BW labeled +4 BW 5/7-5/9 complete ___________ / 5 pts. Page 153- "Formula Mass: Calculating Molecular Mass" notes ___________ / 5 pts. Page 154- "Balancing Equations" Practice ___________ / 5 pts. Page 155- "Balancing Equations" notes ___________ / 5 pts. Page 156- Practice Balancing Equations and Identifying Types of Reaction Bell Work 5/12: Balance the following chemical equations: 1. Na + H2O NaOH + H2 2. C2H2 + O2 CO2 + H2O 3. AgNO3 + K3PO4 Ag3PO4 + KNO3 Bell Work 5/13: Balance the following chemical equations: 1. Ag2O Ag + O2 2. Ba(OH)2 + H3PO4 BaHPO4 + H2O 3. NaOH + H3PO4 Na2HPO4 + H2O Page 157- "Types of Reactions" notes +1 BW labeled +4 BW 5/12-5/13 complete ___________ / 5 pts. ___________ / 5 pts. Page 158- Bell Work 5/14: Balance the following chemical equations: 1. FeS + HCl H2S + FeCl2 2. H3BO3 + K B + KOH Table of Contents pages 132-158 (1pt every 5 pages) ___________ / 5 pts. ___________ / 5 pts. Grand Total __________ / 130 pts.