Water Potential
advertisement

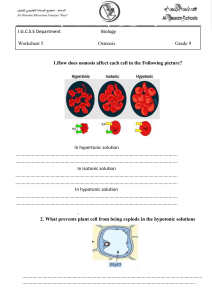
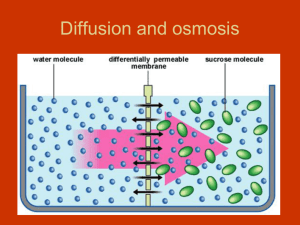
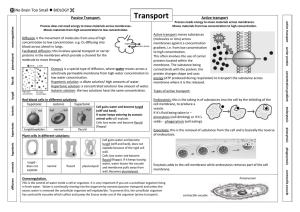
Water Potential (the Greek letter psi, pronounced "sigh") Please read the following carefully and as often as necessary to understand the lab we will be doing this week. Key terms: Water potential, osmosis, hypotonic, hypertonic, isotonic, turgid, flaccid, plasmolysis. Plants must balance the intake of water with water loss. Osmosis is the passive movement of water across a cell membrane. There are openings between adjacent plant cell walls called plasmodesmata (which rhymes with stomata), so osmosis is not impeded by cell walls. Back to water potential: Animal cells lack cell walls, and when placed in a hypotonic solution, water will enter until the cell expands to the point of lysing (bursting). Some unicellular animal-like organisms (protozoan = "first animal") have a subcellular contractile vacuole for releasing the excess water. Plant cells have a rigid cell wall, so they can handle the internal pressure. In fact, they depend on this internal pressure. The internal pressure of plant cells resulting from osmosis is called turgor pressure. Tissues under pressure are turgid. When turgor pressure is too low, owing to moisture stress, the cells and tissues become "deflated" or flaccid. When moisture loss is severe, the plasma membrane separates from the cell wall in a process called plasmolysis. There is a concept that is unique to plants called Water Potential, . Water moves in plants by osmosis from where is higher to where it is lower. is a function of two factors; the solute potential, s, plus the pressure potential, p. So, = S + p. Solute potential is at a maximum at zero (distilled water), whereas pressure potential can be negative (vacuum), zero (one atmosphere), or positive (greater than zero). So distilled water at normal atmospheric pressure has a water potential of zero ( = 0). As solutes are added and their concentration increases, S becomes increasingly negative. Pressure potential (p) is a physical force exerted on the cell wall. As turgor pressure increases, the value for p increases proportionally. Water potential is measured in megapascals (MPa). To get a handle on values of pressure in MPa's, consider that a car tire's pressure is about 0.2 MPa. The pressure of water in your home's plumbing system is about 0.25 MPa. By comparison, most plant cells carry out their various and miraculous functions at about 1.0 MPa! This is equal to about 10X normal atmospheric pressure. So why don't plants explode when you poke a hole in them? It is for the same reason that you can't hear plants "breathe" - the pressure is confined to millions and millions of individual cells which do not release their pressure all at once. Cell walls are amazingly strong. We will be conducting a lab to see if we can find a concentration of sucrose (table sugar) that is equivalent to the solute concentration within plant tissue. Plant membranes are impermeable to sucrose, but permeable to water. Root or tuber tissue works well. Fleshy roots (i.e. carrots, turnips, sweet potatoes) will work, as will tubers (common potatoes are actually modified stems – not roots – called tubers….the scientific name of the potato plant is Solanum tubersosum). Regardless of the solute(s) in plant tissue, their combined concentration will lower the water potential. As pointed out above, net osmosis will occur in the direction from where is higher to where it is lower. Thus, plant tissue placed in a hypotonic solution will take up water, swell, and become more turgid. On the other hand, plant tissue in a hypertonic solution will lose water, shrink, and become flaccid. If we place plant tissues in concentrations ranging from very hypotonic to very hypertonic, we can graph the resulting data and interpolate (intercalate) the concentration that is isotonic. If we graph data (% change in mass vs. concentration), and correlate the data using linear regression (best fit line), we will see that the line will cross the x axis at a point corresponding to y=0.