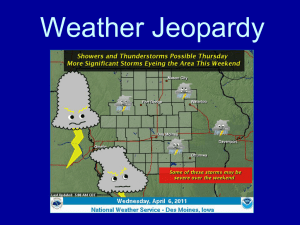
Chapter 4 text
advertisement

4-1 Chapter 4 Water Cycle Water is essential to weather and climate on Earth. The sun supplies energy to Earth in the form of electromagnetic waves. This energy is unevenly distributed over the globe. The atmosphere and ocean transport the excess heat from the tropics to the poles. Much of the heat transported poleward by the atmosphere cannot be measured with a thermometer, but is in the form of latent heat. Solar energy evaporates water from the oceans, the water vapor is transported poleward and when it condenses to form a cloud heat is added to the air. Water continually cycles through the atmosphere as evidenced by the appearance of clouds and precipitation. The formation of clouds and precipitation only occurs under the correct weather conditions, which depend on the wind and water vapor content of the atmosphere. The ability for water to change phase makes it an important energy source for atmospheric storms. Heavy precipitation affects our everyday lives by determining what we wear, hindering transportation, influencing the health of our economy, and even affecting our moods. Every day millions of people listen to weather forecasts to determine what to wear and to plan activities. Certain types of precipitation, heavy snows and freezing rain, are a safety concern in for all modes of transportation. When you complete this chapter you will understand how clouds and precipitation form, how clouds are classified, and the different types of fog. 4-2 Evaporation - the Source of Atmospheric Water Evaporation is the process by which water in its liquid state is converted into water vapor. Evaporation is important to weather and climate because evaporation is the primary route for water molecules to enter the atmosphere. Evaporation occurs because water molecules at the surface of the water have enough energy to escape the liquid. When we heat the water, the molecules move faster, increasing the number of molecules that have enough kinetic energy to escape the main body of water. Eventually, with continued heating, all the molecules in the liquid will have enough energy to enter the vapor phase. Evaporation is an important process in weather and climate studies. Evaporation is greatest when the evaporating surface is much warmer than the air above, the atmospheric pressure is low, the wind speed is high, and there is relatively little water vapor already in the air. Consider the following example of evaporation. A sealed beaker is partially filled with liquid water and Saturation is the condition at which equal numbers of water molecules cross a flat surface between air and water, or air and ice. kept at a constant temperature (Figure 4.1). If the water and the liquid are at the same temperature, the average kinetic energy of water molecules in both the air above and in the liquid is the same. Some individual molecules will have more and some less kinetic energy than the average. For instance, a water molecule in the liquid phase might gain kinetic energy considerably above the average because of several rapid collisions with neighboring molecules. Now imagine this molecule at the liquid's surface, the boundary between the water and the air. If it has enough kinetic energy to overcome the attractive force of nearby molecules and is moving toward the air, it may escape from the liquid. Molecules that escape from the surface form a vapor above the 4-3 liquid. Vapor molecules may collide with the surface of the liquid. If the kinetic energy of one of these molecules is sufficiently below the average, the molecule may be captured and become part of the liquid. At the liquid surface, at any given time, some water molecules will be escaping, or evaporating, and others will be captured, or condensing. If the number evaporating is greater than the number condensing, then the water level in the container lowers. If condensation is greater, the water level rises. Eventually, because the beaker is sealed, the number of molecules leaving the surface of the liquid will be the same as the number captured. There will be no net change in the number of molecules in the liquid phase. A situation in which there is no net change is described as being in equilibrium. When the number of molecules leaving the liquid is in equilibrium with the number condensing, the air above the surface is saturated. Counting the number of molecules in the beaker above the water is one way to measure the amount of water in the beaker's air. There are several methods of specifying the amount of water vapor in the atmosphere. Measuring Water Vapor in the Air Specifying the amount of water vapor in the atmosphere is important for several reasons: 1. Water is the only substance that can exist in all three phases (vapor, liquid and ice) in the atmosphere. The change of phase of the water is an important energy source for storms and atmospheric circulation patterns. The change of phase of water also affects the buoyancy of air, which is important in cloud and precipitation formation. 4-4 2. Water vapor is the source of all clouds and precipitation. The potential for cloud formation and dissipation depends on the amount of water vapor in the atmosphere. 3. The amount of water in the atmosphere determines the rate of evaporation. Rates of evaporation are important for weather and to many forms of plant and animal life. 4. Water vapor is a principle absorber of shortwave and longwave radiant energy. It is the most important greenhouse gas. News reports of current weather conditions often include the dew point temperature and the relative humidity. These are just two of several ways to express the amount of water vapor in the atmosphere. Each is a method that has advantages and disadvantages. In this section we will discuss four different methods of representing the amount of water vapor in the atmosphere: mixing ration, vapor pressure, relative humidity and dew point/frost point. In addition to memorizing the definitions associated with these methods, it is important to learn how these methods of describing the amount of water in the atmosphere change when temperature and pressure conditions vary. Mixing Ratio One way of expressing the amount of water vapor in the atmosphere is the ratio of the weight of water vapor to the weight of the other molecules in a given volume of air. This is the mixing Mixing ratio expresses the amount of water in the atmosphere in terms of the mass of water vapor per unit mass of dry air. ratio. The unit of mixing ratio is grams of water vapor per kilogram of dry air (g/kg). Typical values of the mixing ratio near the surface of the earth range between less than 1 g/kg in polar regions to over 15 g/kg in the tropical regions. 4-5 Since the surface of the Earth is a source of water vapor for the atmosphere, the mixing ratio generally decreases the farther you get from the surface (Figure 4.2). Adding or removing water vapor molecules from a fixed volume of air changes its mixing ratio. Evaporating water into the volume increases the mixing ratio. Since missing ratio has to do with weight, as opposed to temperature or volume, cooling the air or expanding the air has no effect on the value of the mixing ratio, since the total mass and total number of molecules remain unchanged. As you learned in Chapter 2, the amount of solar energy absorbed by the atmosphere is related to how many water molecules are present relative to other molecules. For this reason, when discussing radiative energy transfer in the atmosphere, the amount water vapor is often expressed in terms of the mixing ratio. Vapor Pressure Gas molecules exert a pressure when they collide with objects. The pressure the water molecules exert is another useful method of representing the amount of water vapor in the atmosphere. There is always water vapor in the atmosphere. The pressure exerted by these water vapor molecules is the vapor pressure. Atmospheric vapor pressure is expressed in millibars (mb). The number of water vapor molecules in the atmosphere is always small compared to the number of nitrogen and oxygen molecules, so the vapor pressure is small Vapor pressure measures how much water vapor is in the atmosphere in terms of its pressure. compared to the total atmospheric pressure. Near the surface of Earth the vapor pressure is typically less than 40 mb while the average atmospheric pressure is approximately 1013 mb. 4-6 A variety of things can change the vapor pressure. The higher the temperature, the greater the average kinetic energy of the molecules and the higher the vapor pressure. Increasing the number of water vapor molecules for a specific volume of air will also raise the vapor pressure. If more water evaporates into a volume of air, both the vapor pressure and the mixing ratio increase. However, if we cool the air, the vapor pressure decreases, but the mixing ratio remains constant. Atmospheric scientists use vapor pressure to express the amount of water in the atmosphere when they discuss the formation of cloud particles. When air is saturated (as in Figure 4.1), the pressure exerted by the water vapor molecules is called the saturation vapor pressure. Remember that the ability of a molecule to escape from the intermolecular forces in the liquid is a function of its kinetic energy. As the temperature of water increases, the number of molecules with enough kinetic energy to evaporate from the water surface increases. Increasing the temperature increases the number and speed of the water molecules in the vapor phase. More molecules moving at greater speeds exert more pressure. Therefore, the saturation vapor pressure increases Saturation vapor pressure is the vapor pressure at which the number of molecules leaving a flat liquid, or ice, surface equals the number of molecules entering the liquid or ice. It is a function of temperature. as the temperature increases. Saturation vapor pressure in the atmosphere is reached whenever the atmospheric water vapor exerts a pressure equal to what the saturation vapor pressure would at that particular temperature in a closed container. 4-7 Relative Humidity Neither the vapor pressure nor the mixing ratio tells us how close the air is to being saturated. The ratio of the actual vapor pressure exerted by molecules of water vapor to the saturation vapor pressure at the same temperature is an indication of how close the air is to Relative humidity indicates how close the air is to saturation. saturation and is called the saturation ratio. Multiplying the saturation ratio by 100% yields the relative humidity. Saturated air has a relative humidity of 100%, since the vapor pressure equals the saturation vapor pressure. A relative humidity of 50% tells us the vapor pressure is half that required for saturation. Relative humidity can exceed 100% by a few tenths of a percent. This is referred to as supersaturation. Relative humidity describes how far the air is from saturation. It is specifically used to express the amount of water vapor when discussing the amount and rate of Supersaturated conditions represent relative humidities of greater than 100%. Relative humidities of greater than 100.4% are rare. evaporation. Relative humidity is also commonly mentioned during weather reports because it is an important indicator of the rate of moisture and heat loss by plants and animals (See Box 4.1). Changing the vapor pressure changes the relative humidity. Adding water molecules to a fixed volume of air increases the vapor pressure but has no effect on the saturation vapor pressure. Adding water molecules to a volume of air increases the relative humidity. A dehumidifier lowers the relative humidity of the air by removing water vapor molecules from the air. 4-8 Changing the saturation vapor pressure also changes the relative humidity. The saturation vapor pressure decreases if the temperature of the air decreases. Therefore, a decrease in temperature results in an increase in the relative humidity. Dew Point/ Frost Point So, one way to approach saturation, a relative humidity of 100%, is to cool the air. In order to do that we need to know how much the air needs to be cooled to reach saturation. When air near the ground is saturated, water condenses on objects to form dew (Figure 4.3). So, the temperature to which air must be cooled to become Dew point is the temperature to which air must be cooled at constant pressure to become saturated. saturated without changing the pressure is called the dew point. The dew point temperature is determined by keeping the pressure fixed because changing the pressure affects the vapor pressure and therefore the temperature at which saturation occurs. The dew point temperature tells us nothing about how many water molecules are in the atmosphere or how close the air is to a relative humidity of 100%. To know how close the air is to saturation, we need to know the dew point and the air temperature. The dew point temperature can never be greater than the air temperature. When the dew point equals the air temperature, the air is saturated. The closer the dew point is to the air temperature, the closer the air is to saturation. The temperature difference between the air and the dew point temperature is called the dew point depression. If the temperature to which air must be cooled at a constant pressure to become saturated is Frost point is the highest temperature at which atmospheric moisture will form frost. below 0C (32F), that temperature is called the frost point. 4-9 Whether or not a blade of grass cools below the frost point is a function of its energy gains and losses. On clear nights, objects (such as blades of grass) loose energy by radiative processes. Grass loses energy by emission of longwave radiation while gaining energy by absorbing the longwave radiation emitted from surrounding objects. Under clear sky conditions, more radiation is emitted by the objects on the ground than the sky, and so the blades of grass cool. If the temperature of a grass blade falls below the frost point, frost will form on the grass. There are many occasions when frost forms in an open field but not under a tree (see Figure 4.4.) This is because trees emit more radiation towards the ground than the clear sky. Energy losses of the grass in the open field are greater than the grass under the tree. The grass in the open field cools faster and reaches the frost point before the grass blades under the tree. Certain conditions are favorable for dew and frost to Dew is water that condensed onto a form. First, dew and frost form in air close to the ground. surface near the ground that has fallen below the dew point. Frost Radiative processes cool the surface to the dew point forms if the dew point is below freezing. temperature. The object at the surface on which the dew will form must be effective at emitting longwave radiation. As the surface is cooling it must be insulated from receiving heat from the soil. A high relative humidity in the surface air layer and a low specific humidity of the air above the surface is favorable for dew formation as these conditions permit sufficient cooling of the object. Dew forms when air reaches the dew point. Dew may form and then freeze if the temperature falls below freezing forming frozen dew. Frozen dew is different from frost. The dew point is useful in forecasting minimum temperatures, forecasting the formation of dew and frost, and predicting fog. Formation of frost and dew are examples 4-10 of phase transitions between the gas phase of water and its solid and liquid states. Cloud formation is another example of a phase transition. Condensation and Deposition - Cloud Formation Clouds form when the water vapor condenses into small particles that can either be liquid or solids. Liquid particles suspended in the atmosphere are referred to as cloud droplets and the solid particles are often called ice crystals. This section discusses how vapor can change phase to form cloud droplets and ice crystals. Water vapor molecules are always condensing onto surfaces. They are also always leaving these surfaces. If condensation is greater than evaporation a thin film of water will form on an object. Comparing the rate of condensation relative to evaporation is another way to think of relative humidity. A relative humidity of 100% means that condensation equals evaporation, while a 90% relative humidity means that condensation is less than evaporation. A relative humidity of greater than 100%, which often occurs in clouds, means that condensation is greater than evaporation! As a volume of unsaturated air cools, its relative humidity increases. If sufficiently cooled, the relative humidity becomes 100%, the temperature equals the dew point and it seems that condensation and cloud formation should occur. But forming cloud droplets can actually occur at relative humidities of more than 100% and less than 100%! Why? Because of the opposing forces of the curvature effect and the solute effect. Relative humidity is measured with respect to a flat surface. In our previous discussion of evaporation we discussed a single molecule near the edge of this flat surface of still water. This molecule is attracted by its neighbors, which attempt to keep it part of 4-11 the water. For the surface molecule to escape the water it must have enough energy to overcome the attractive forces of the surrounding water molecules. But what if the surface is curved, like that of a water droplet? A molecule on the surface of a drop of water has fewer neighbors to attract it (Figure 4.5) and can therefore escape the fluid more easily. The smaller the droplet, the fewer the neighbors and the easier it is for a water molecule on the surface to escape. If the air is saturated with respect to a flat surface of water, it is unsaturated with respect to a curved surface. This is called the curvature effect. It opposes the formation of small droplets by condensation. Adding salt to water suppresses evaporation. Salt dissolves in water and some of the salt molecules end up at the surface, replacing water molecules. Salt molecules do not vaporize like water molecules. The presence of salt molecules at the surface physically interferes with the escape of water molecules. The greater the concentration of salt, the greater evaporation is reduced, all other things being equal. This is called the solute effect and it enhances the growth of droplets by condensation by suppressing evaporation. But as the droplet grows the solution becomes more dilute and the solute effect decreases. The solute and curvature effects oppose one another. They are important in the initial growth of cloud droplets. Their combined effects help determine which cloud droplets grow and which dissipate. But both effects weaken as the drop size increases and finally become negligible for droplets with radii larger than about 5 microns. Rain drops all start out small and grow large. For a cloud droplet to grow requires that the rate of evaporation be less than the rate at which condensation is occurring. How they grow is the subject of this section and the next. 4-12 It is not easy to spontaneously form a water droplet. For water vapor molecules to form a tiny cloud droplet of pure water requires a relative humidity of more than 200%. This is because a tiny droplet has a strongly curved surface. Relative humidities this high are not observed in the atmosphere. The initial formation of a cloud droplet around any type of particle is called nucleation. There are two types of nucleation, homogeneous and heterogeneous nucleation. Homogeneous nucleation means that the Nucleation is the initial process of droplet or ice crystal formation. droplet or ice crystal is formed only by water molecules. In homogeneous nucleation enough water molecules have to bond together to form a cluster that then acts as a nucleus for further condensation. Water-only bonding only works if the water molecules to have low kinetic energy. If the kinetic energy of the molecules is too high the cluster quickly evaporates. For this reason, homogeneous nucleation only occurs at temperatures colder than -40C (-40F). Clouds form in air that is warmer than -40C (-40F), so cloud formation requires a different type of nucleation. Heterogeneous nucleation happens when small, non-water particles serve as sites for cloud droplet formation. The particles are usually aerosols and the aerosols that assist in forming liquid droplets are called condensation nuclei. In the next sections, we will first consider the formation of liquid droplets around condensation nuclei and then address how ice crystals form around ice nuclei. Condensation nuclei There are two types of condensation nuclei: hygroscopic and hydrophobic. Hygroscopic nuclei dissolve in water while hydrophobic nuclei do not. Condensation is 4-13 more favorable on hygroscopic nuclei. Hygroscopic nuclei allow water to condense onto the nuclei even when the relative humidity is below 100% because of the solute effect. There are plenty of condensation nuclei in the atmosphere in the form of dust, salt, pollen and other small particles. The surface of the Earth is a source of aerosols and so the concentration of condensation nuclei is, generally speaking, greatest near the surface and decreases with altitude. Variations in the numbers and types of condensation nuclei exist over different geographical regions. Over the oceans the air has fewer condensation nuclei than over land. Many of the nuclei over the oceans also contain salt, making them hygroscopic nuclei. Tiny salt-water droplets are thrown from the ocean's surface by waves and whipped from the surface by the winds. The water evaporates leaving behind a salt particle. Cities have more condensation nuclei than pristine environments far from humans. In general, there is no deficiency of condensation nuclei for forming water droplets. Ice nuclei When ice crystals form, water molecules can not deposit onto the crystal haphazardly, as they can Ice nucleus is a particle that serves as a nucleus in the formation of an ice crystal in the atmosphere. when condensing onto an existing water droplet. The molecule must be accommodated into the structure of the crystal. Ice nuclei or the particles around which ice crystals form, are important in the embryonic stage of an ice crystal because they make it easier for deposition to occur. There are four methods of ice particle formation: deposition nucleation, freezing nucleation, immersion nucleation, and contact nucleation. In deposition nucleation, water vapor molecules enter the ice phase directly on a deposition nucleus when the air is supersaturated with respect to ice. Deposition nuclei 4-14 are particles with a molecular geometry resembling the lattice structure of ice. The geometry helps water molecules to align in the proper molecular structure for ice when they deposit on the surface of the nuclei. Clay particles are naturally occurring objects that serve as ice nuclei. Silver iodide can also serve as an effective deposition nucleus. Freezing nucleation is the process by which a super-cooled drop freezes without the aid of a foreign particle. Liquid water with a temperature below 0C is referred to as super-cooled water. There are differences in the freezing temperatures of a small water droplet and a large body of water. The freezing point of a large body of water (such as the water in your ice tray) is 0C (32F) at standard pressure. Small droplets do not freeze at this temperature. For example, a 1-millimeter diameter droplet will generally not freeze until the temperature falls below -24F (-11C). Why is this? For ice to form all the water molecules must align in the proper crystal structure. First a few molecules align, and then the rest quickly follow, turning the liquid into a block of ice. The larger a volume of water is as its temperature falls below freezing, the higher the chances that a few of the molecules will line up in the proper manner. In a small volume of water the chances of some of the molecules aligning in the correct structure is reduced, simply because there are fewer molecules. Contact and immersion nucleation both cause ice crystallization by lowering the freezing temperature of a super-cooled droplet. Unlike deposition nucleation, which forms an ice crystal directly by deposition, contact and immersion nucleation each cause a liquid drop to freeze and become a small ice crystal. In immersion nucleation the ice nuclei is submerged in the liquid drop. Once the drop reaches a given temperature, the immersed ice nucleus allows the super-cooled 4-15 liquid to rapidly align in the crystalline structure of ice, causing the drop to freeze. Different ice nuclei become active at different temperatures. For example, if silver iodide is submerged, a droplet that is cooled to a temperature of about -4C (25F) will freeze. Ice nuclei may also collide with super-cooled drops. Contact immediately freezes the drop. This is referred to as contact nucleation. Particle Growth by Condensation and Deposition Once a cloud particle forms it can grow in a saturated environment. If the particle is a liquid droplet, it will continue to grow by condensation, if the vapor pressure of the air is greater than the vapor pressure just above the surface of the particle. If the particle is ice, then it grows by deposition. Growth by condensation and deposition both involve diffusion and can be referred to as growth by diffusion. Remember that diffusion is a molecular process that results because the molecules are moving. Diffusion is fast on very small scales and is slow on large scales. For this reason growth by diffusion is fast when the cloud particles are small. As the cloud particle gets larger, diffusion becomes less important and the growth by condensation and deposition of water molecules slows. So growth by condensation and deposition produces small droplets, but takes a long time to create droplets large enough to fall as precipitation. (The formation of precipitation sized particles is discussed later in the chapter.) While growth by condensation cannot produce a rainstorm, the process is important in the formation of clouds or fog. Fog Formation Fogs form when air near the ground becomes saturated, activating cloud condensation nuclei which then form a suspension of tiny water drops. The air near the 4-16 ground can become saturated as it cools or when water from the surface evaporates into it. The formation of heavy fog often reduces visibility to the point where certain modes of transportation become Fog is a cloud that is in contact with the ground. hazardous. The appearance of a fog on a highway can lead to a chain-reaction of accidents that can involve scores of vehicles. Fog also played a role in the collision between the Titanic and an iceberg. In early December 1952 a fog in London became so thick, partly because of pollution, that people walked into canals and rivers because they could not see the ground! The distribution of heavy fog over the continental United States is given in Figure 4.6. The frequency of heavy fog in Alaska, Hawaii and Puerto Rico is generally less than 10 days per year. Fog is common in the Appalachian Mountains, the northwest and northeast coastal regions. The different types of fog are named after the method by which saturation is reached and are called: radiation fog, advection fog, mixing fog, evaporation fog and upslope fog. Radiation Fog Surface radiation fogs form on clear, long nights as the ground rapidly cools by radiation and the air just above the ground cools by conduction and radiation (Figure 4.7). As the temperature of the air drops, the relative humidity increases. Since radiative cooling near the ground is more rapid on clear nights, radiation fogs tend to develop on clear nights. Light winds are also required because they can gently mix moist air near the ground. Winds that are too strong mix the air near the ground with the drier, warmer air above keeping the air near the surface from saturating. 4-17 Radiation fogs are frequent in the fall in river valleys and small depressions. The cold air sinks to the bottom of the valley providing the cool air, while rivers and streams provide the water vapor needed to increase the relative humidity via evaporation. These fogs are often called valley fogs. There are a couple of rules for forecasting a radiation fog. If the dew point temperature is approximately 5C below the air temperature at sunset, and if the winds are predicted to be less than 5 knots, there is a good chance that a radiation fog will form during the night. Advection Fog When warm air is advected over a cold surface, the air near the ground cools due to energy exchanges with the surface, the relative humidity increases, and an advection fog may form (Figure 4.8). Advection fog is common off the coast of California as warm moist air over the Pacific is advected over the cold coastal waters. Off the east coast, warm air over the coastal waters of the Gulf Stream may be advected over the cold Labrador current. Cold air may also be advected from New England to over the warm coastal waters of the Gulf Stream. These fogs form at all times of the year and can last for more than a week. Another foggy region is off the coast Japan, where the cold water of the Oyoshio current meets the warm Kuroshio current. Advection fogs can also occur when warm air flows from over the water to cooler land. Fog is common along the coast of the Gulf of Mexico during fall and winter. During these times saturation of the air occurs when warm moist air flows from the Gulf of 4-18 Mexico over the cooler land. Fog formation quickens as the air cools by radiation. These types of fog are also common in New England. Mixing Fog It is possible to form a mixing fog by mixing two air parcels that are each unsaturated. Mixing the two air parcels results in a single air parcel that is saturated. A good example of a mixing fog is when you see your breath on a cold day. Warm moist air from your lungs mixes with the cooler drier air of the environment. The mixed air cools rapidly to the saturation point and the water vapor condenses. Mixing fogs also form over lakes at times when air is much colder moves over warmer water. The vapor pressure of the cold air is less than that of the air just over the water so evaporation is rapid. This rapid evaporation saturates the air above the surface. The condensation further warms the air and the warmed air rises and mixes with the cold air above causing more fog to form. This type of fog gives the appearance of steam rising out of the water and is sometimes referred to as a steam fog (Figure 4.9). It is common over lakes during late autumn or early winter in the higher midlatitude regions of the globe. Steam fog is frequent when very cold air rushes over unfrozen waters. Evaporation Fog If you take a long hot shower, you may “fog up” the bathroom. Some of the warm water from the shower evaporates into the cooler bathroom air, moistening it to saturationforming a fog. Evaporation fogs also occur in the vicinity of warm fronts and are sometimes called frontal fogs. These fogs form when water evaporates from rain that falls from warmer air above the ground into cold air near the surface. Frontal fogs only 4-19 form after it has been raining for hours because it takes time for the evaporating drops to saturate the air. Similarly, it is difficult to fog up the bathroom by taking a short shower. Upslope Fog Consider air rising over a mountain barrier. As the air rises, it expands, cools, and approaches saturation creating an upslope fog. Upslope fog is common in moist mountainous regions such as the Appalachian Highlands. This type of fog forms best when the air near the ground, before flowing upslope, is cool and moist. This way it does not have to be lifted too high before saturation occurs. Lifting Mechanisms to Form Clouds Fog is classified by the method the air reached saturation. The formation of a cloud also involves humidification of the air. Most clouds form when air cools to the dew point as a parcel of air rises. As parcels of air rise, they expand because atmospheric pressure decreases with altitude. Expanding the parcel requires work, which requires energy. This energy comes from decreasing the kinetic energy of the molecules inside the parcel. As it rises the parcel's temperature decreases at a rate described by the dry adiabatic lapse rate, 10C per 1000 meters lifted. As the parcel of air continues to rise, it cools and the relative humidity increases and, assuming that there are cloud condensation nuclei in the parcel, cloud particles form. To get a cloud to form, the parcel of air must be lifted to an altitude, or level, at which Lifted Condensation Level is the level to which air must be lifted for condensation (or deposition) to occur. condensation occurs. This altitude is called the lifted condensation level (abbreviated LCL). Lifting a parcel of air above the height of the lifted condensation level results in the formation of a cloud. 4-20 Figure 4.10 depicts four mechanisms that cause air to ascend. Air is lifted as it moves against a mountain range (Figure 4.10a). The air cannot penetrate this physical barrier and so it has to flow over the mountain. This is orographic lifting. Barriers of air also exist in the atmosphere and are caused by differences in density. For example, at the same pressure, cold air is more dense that warm air. Fronts represent the boundaries between these air masses of different densities. As fronts move, less dense warm air is forced to rise over the cooler, more dense air. Warm frontal lifting occurs when a warm air replaces cooler air. Cold frontal lifting occurs at cold fronts. Warm and cold frontal lifting are An updraft is a current of air that has a marked upward vertical motion. Updrafts keep cloud particles suspended in the air. demonstrated in Figure 4.10b. Frontal lifting is common in winter. During summer, convection is an important lifting mechanism. In summertime convection, solar energy passes through the atmosphere and heats the surface. The air near the surface warms, becomes less dense than the air around it and so rises (Figure 4.10c). The final mechanism, convergence, occurs when air near the surface flows together from different directions. When the air near the ground converges, or is squeezed together, it moves upward. Each of these cases the rising air creates an updraft. Certain atmospheric conditions are less favorable for lifting a parcel of air than other conditions. For example, temperature inversions and stable atmospheres suppress atmospheric vertical motions. As the parcel rises its temperature decreases and, since the atmosphere is warming with altitude, the parcel becomes colder, and thus more dense than its environment and so sinks. Unstable atmospheres on the other hand are very favorable for cloud and precipitation formation, because unstable atmospheres are susceptible to rising motions. 4-21 Cloud Classification Fog types were classified based on the process that caused the air to become saturated. Clouds are classified according to their altitude and their appearance. Luke Howard (a pharmacist) derived the cloud classification system commonly used today in 1803. According to this classification system, there are two fundamental cloud structures layered or convective and altitude. Clouds are also categorized based on whether or not they are precipitating. Layered clouds have a horizontal extent that is much larger than their vertical depth. Layered clouds generally have flat bases and tops and can extend from horizon to horizon. The term stratus describes the layered cloud category. Stratus clouds result when a layer of air is gradually lifted over a large region. Convective clouds have a vertical depth that is the same or often larger than their horizontal extent. The appearance of convective clouds are often described with words such as lumpy, cellular, piled up, or cauliflower. Convective cloud types are indicated by the root word cumulo. Convective clouds may become very tall and are rounded on top. They generally form in unstable air. Clouds are also classified by their altitude above the surface. The root word cirro describes a high cloud. The root words alto and strato are used to indicate a middle and low cloud, respectively. The approximate cloud base heights that demark these different cloud types are given in Table 4.1. The root words used to describe the appearance (stratus and cumulo) and height (cirro, alto, and strato) of a cloud are combined to indicate the cloud type. For example, a high cloud that is layered is called a cirrostratus. While a layered cloud at middle levels of 4-22 the troposphere is called an altostratus. A layered cloud with a low cloud base is just called a stratus, not a stratostratus. It is also useful to distinguish clouds that are raining from those that are not. The root nimbus indicates that precipitation is falling from the cloud. Thus, nimbostratus is a layered cloud that is raining. Cumulonimbus is a towering cloud that is raining. The main cloud types we will discuss in this chapter are shown in Figures 4.11. Stratus (St) Stratus clouds, abbreviated St, are low altitude clouds, which are diffuse in appearance (Figure 4.12). From the ground they appear as light to dark gray in color and cover the sky. They are common along coastlines and in valleys. Early morning fogs may lift and form a stratus deck. Stratus clouds may also originate when moist, cold air is advected at low altitudes over a region Stratocumulus (Sc) Stratocumulus (Sc) are low-lying clouds that cover the sky and appear white to gray in color (Figure 4.13). Unlike stratus, stratocumulus have more structure that appears as variations such as rows or patches. Stratocumulus are distinguished from stratus by these variations in color across the sky. As with stratus, stratocumulus are frequent in certain geographic regions, like along coasts and in valleys. Cold air passing over warmer water will also produce stratocumulus. This is why this cloud is common in the winter near the Great Lakes. Stratocumulus also accompany cold fronts. When associated with a large weather system, stratocumulus are often the last cloud to appear before the skies clear. 4-23 Nimbostratus (Ns) Nimbostratus (Ns) are deep clouds that bring precipitation and appear dark gray to pale blue in color (Figure 4.14). The structure of the base is ill defined and so nimbostratus sometimes look similar to stratus, stratocumulus or altostratus. The difference is that nimbostratus produce precipitation. Nimbostratus are often associated with the passage of warm fronts. Cumulus (Cu) Cumulus clouds (Cu) generally have well-defined, flat bases and domed tops resembling cauliflower. The edges of the cloud are distinct. The bases are generally dark and the sunlit sides bright white. These clouds form whenever rising air associated with large-scale weather systems has a high relative humidity. The height of the cloud base is related to the relative humidity of the rising air. Cumulus clouds in the dry southwest US generally have higher bases than those from in the southeast. Cumulus clouds may also form over mountains or large hills if the air is unstable. These orographically forced clouds appear stationary though they continually form and dissipate. There are two basic forms of cumulus clouds, fair weather cumulus and cumulus congestus. Fair weather cumulus clouds (Figure 4.15) have a height similar to their width. These clouds are common in summer when air rises due to convection that comes from solar heating of the surface. During autumn and winter cumulus form over large open lakes. Fair weather cumulus are not deep enough to cause rain, though some may grow into large storms. Cumulus congestus or towering cumulus have a vertical depth that is greater than their width. For them to form the atmosphere must have a deep unstable layerdeeper 4-24 than what is required for the formation of the fair weather cumulus. Cumulus congestus tilt if the wind speed increases with altitude. These towering clouds are common in summer and may have light rain falling from them. In regions like Florida, cumulus congestus may produce heavy rains for a few minutes. When cumulus congestus form in the morning it is a good indicator that storms may form later in the day. If the tops appear fuzzy, ice is forming and the cloud may be developing into a cumulonimbus. Cumulonimbus (Cb) Cumulonimbus (Cb) reach to high altitudes, often to the tropopause, and produce large amounts of precipitation. The tops of cumulonimbus are often seen on the horizon while the bases are not in view (Figure 4.16). A distinguishing feature of the cumulonimbus clouds is its anvil shaped top. This is the part of the cumulonimbus that appears flat near the top and extends a long distance downwind from the central updraft of the cloud. This flat part of the cloud is similar in shape to the anvil used in metal smithing. Cumulonimbus clouds form in unstable, moist atmospheres and are common in spring and summer. They often occur in the advance of a cold front. In summer they can form over mountains due to orographic lifting in combination with solar heating. Precipitation falls from these clouds and severe weather (lightning, hail, tornado, and flash flood) is common. Cumulonimbus can be isolated storms or organized in groups. When cumulonimbus clouds form together in an organized system, the chance of severe weather increases. 4-25 Altostratus (As) Altostratus (As) are layered clouds consisting mostly of liquid water and are gray to pale blue in appearance (Figure 4.17). Altostratus form when the middle layers of the atmosphere are moist and gradually lifted. The cloud base does not have much structure. If the sun appears through these clouds, it has a “watery” appearance. The height of the cloud base is hard to distinguish because of its smooth appearance. Altostratus clouds are common in the advance of a warm front, preceding the nimbostratus. Altocumulus (Ac) The appearance of altocumulus (Ac) varies considerably. They can be thin or thick, white or gray, occur in organized lines or random distributions. They occur in the middle levels of the atmosphere when the air is moist. They are similar in appearance to stratocumulus, though with a higher cloud base and generally do not fill as large a part of the sky (Figure 4.18). A “mackerel sky” is one that contains altocumulus clouds. Altocumulus clouds appear in the advance of a warm front, preceding the altostratus. If the altocumulus clouds appear with other cloud types, an approaching storm is likely. Altocumulus occur frequently and are common in many places of the world. Cirrocumulus (Cc) Cirrocumulus clouds (Cc) are thin, white clouds that appear in ripples arranged in a regular formation (Figure 4.19). They are distinguished from altocumulus by the smaller size of the individual cloud elements. They occur high in the atmosphere and are composed of ice crystals and indicate a moist layer. While these clouds occur year round, they are not very frequent and are usually present with other cloud types. Cirrocumulus are often associated with large weather systems. 4-26 Cirrostratus (Cs) Cirrostratus clouds (Cs) can cover part or all of the sky. They are uniform in appearance and can be thin or thick, white or light gray in color (Figure 4.20). They occur high in the atmosphere and are composed of ice crystals. They are common during winter in association with large-scale weather systems. If the cirrostratus cloud thickens into an altostratus, it is indicative of an approaching weather disturbance. They may also appear far out in advance of a tropical or subtropical weather disturbance. Cirrostratus often form thin veils through which the sun can easily be viewed. Halos around the sun, bright arcs, and brilliant spots are optical phenomena associated with sunlight passing through the ice crystals composing the cirrostratus, topics of the next chapter. Cirrus (Ci) Cirrus (Ci) are wispy, fibrous, white clouds that are composed of ice (Figure 4.21). They are a common cloud type that occurs in vertical motions ahead of large-scale weather patterns. Cirrus clouds are associated with all weather systems. They can also be generated by flow over mountains or in regions with strong winds, such as the jet stream. They often occur as wisps aligned in the same direction and generally do not completely cover the sky. When isolated cirrus occur, they are not indicative of a weather disturbance. Cirrus clouds similar in shape to a comma are called cirrus uncinus or mares tails. The classification of clouds is based on their appearance and altitude. The appearance and altitude of a cloud is dependent on the lifting mechanism. Besides the appearance and altitude of a cloud, there are also differences in the size and shape of the particles that compose these different cloud types. 4-27 Cloud Composition When we concern ourselves with the particles that compose clouds, we study the microphysical properties of the cloud. The microphysical properties of a cloud include the number of particles in the cloud, how many different sizes exist in the cloud, and the shape of the ice particles. Differences exist between the number of droplets in clouds that form over land and those that form over the oceans. There are more cloud condensation nuclei over land than the oceans. So, continental clouds tend to have a greater number of drops than maritime clouds. Clouds over land have approximately 500 million to 1 billion cloud droplets be cubic meter of air. Maritime clouds typically only have 50 to 100 million droplets per cubic meter of air. But since the water content of maritime and continental clouds are similar, the drops in continental clouds are, on average, smaller and more numerous than the maritime counterparts. Maritime clouds have large, soluble heterogeneous nuclei, which favors the formation of large droplets. Cirrus clouds are composed of ice crystals. The number and sizes of crystals in a cirrus cloud are extremely variable. The Crystal habit refers to one of several characteristic forms of ice crystal. size and shape, or crystal habit, the crystal forms is related to the temperature and supersaturation of the environment in which the particle grows. This relationship between habit and the environmental conditions has been determined by growing ice crystals in a laboratory. Ice crystals can be grown in a laboratory using chambers to control the environmental temperature and humidity. The crystal habit is primarily determined by the temperature of the environment that the crystal grows in, while the rate of growth is determined by the available moisture supply. 4-28 There are five basic shapes of ice crystals, plate, column, dendrite, flat plate and needle (Figure 4.22 include temperature growth). When the temperature of the crystal environment is between 0 and -3 C (32 to 27 F) the preferred crystal form is a thin hexagonal plate. The hexagonal plate also forms and grows at temperatures ranging between -16C and -25C (3F to -13F). The needle, a very long, thin sliver of ice, is the preferred habit when the temperature is between -3 and -5 C (23 to 27F). A prism is a shape similar to a six sided pencil and is the preferred habit in temperature between -5C to -8C (23F to 17F) and -25C to -50C (-13F to -58F). Dendritric shaped crystals are hexagonal with elongated branches, or fingers, of ice. Dendrites are the preferred growth habit at temperature between -12C and -16C (3 to 10 F). Clouds and the greenhouse effect As you learned in Chapter 2, greenhouse gases, such as water vapor and carbon dioxide, warm the atmosphere by absorbing the heat emitted from the surface. Water vapor is the most important greenhouse gas because it absorbs longwave energy effectively. Increases in the greenhouse gases with time can potentially result in a climate change, as the atmosphere becomes more effective at absorbing longwave energy emitted by the surface. As the air temperature warms, the relative humidity initially decreases. Evaporation depends on relative humidity. So as the atmosphere warms, more evaporation occurs, which adds more water molecules to the atmosphere and enhances the greenhouse warming. The Earth and atmosphere keep heating up until the heat 4-29 emitted balances the amount of sunlight absorbed. But greenhouse gases are not the whole story to this scenario of climate change. Clouds have a large impact on the solar and terrestrial energy gains of the atmosphere. Clouds reflect solar energy and reduce the amount of solar radiation reaching the surface, and thus cause a cooling of Earth (Figure 4.23). The thicker the cloud, the more energy reflected back to space, and the less solar energy available to warm the surface and atmosphere below the cloud. By reflecting solar energy back to space, clouds tend to cool the planet. Clouds are also very good emitters and absorbers of terrestrial radiation. Clouds block the emission of longwave radiation to space and inhibit the ability of the planet to emit its absorbed solar energy to space in the form of longwave radiation (Figure 4.23). Thus, in the longwave, clouds act to warm the planet, much like the greenhouse gases do. To complicate matters, the altitude of the cloud is important in determining how much they warm the planet. Cirrus are cold clouds. Thick cirrus therefore emit very little to space because of their cold temperature, while at the same time cirrus are effective at absorbing the surface emitted heat. Thus, with respect to longwave radiation losses to space, cirrus tend to warm the planet. Stratus also warm the planet but not as much as cirrus. This is because stratus are low in the atmosphere and have temperatures that are more similar to the surface than cirrus clouds. Stratus absorb radiation emitted by the surface, but they emit similar amounts of terrestrial radiation to space as the surface. To complicate matters still further, how effective a cloud is at reflecting sunlight is a function of how large the cloud droplets or cloud ice crystals are! We will investigate the reasoning behind this in Chapter 5, but it is easily demonstrated with some broken 4-30 glass. A pile of pieces of small glass is brighter, more reflective, than a pile of large pieces of glass. Clouds consisting of small droplets are brighter than clouds consisting of large particles. Thus, clouds composed of small particles cause more of a cooling than clouds with large particles. So, clouds can either act to cool or warm the planet, depending on how much of the Earth they cover, how thick they are, how high they are, and how big the cloud particles are. Recent measurements by NASA indicate that on average, clouds' reflection of sunlight dominates over the clouds' greenhouse warming. Thus, today's distribution of clouds tends to cool the planet. But this may not always be the case. As the atmosphere warms the distribution of cloud amount, cloud altitude, cloud thickness may all change. We do not know what the effect of clouds will be on the surface temperatures if the global climate changes. Clouds could dampen any greenhouse warming by increasing cloud cover or decreasing cloud altitude. On the other hand, clouds could increase a warming if the cloud cover decreases or cloud altitude increases. Climate is so sensitive to how clouds might change that an accurate prediction of climate change hinges on correctly predicting cloud formation. As you shall see in the coming chapters, predicting clouds is a difficult task. Predicting precipitation is just as difficult. Precipitation Precipitation is any liquid or solid water particle that falls from the atmosphere and reaches the ground. Precipitation is any liquid or solid water particle that falls from the atmosphere and reaches the ground. Because precipitation is formed from water vapor, it removes water vapor from the atmosphere. Rain, snow, sleet, freezing rain and hail are all 4-31 forms of precipitation. Dew and frost also remove water vapor through condensation, or deposition, onto surfaces on the ground. In precipitation, water vapor condenses onto a particle that eventually grows large enough to fall out of the cloud and to the surface. After discussing the growth of precipitation sized particles, we'll talk about rain, freezing rain, sleet, snow, and hail. Growth of precipitation size particles If the particles in a cloud grow large enough, gravity overcomes vertical updrafts and the water particles fall as precipitation. But before discussing precipitation, we have to discuss how the particles become large enough for the gravitational attraction to overcome the updraft forces that are attempting to keep the particle in the air. Cloud particles are typically 10 microns in size, while small raindrops are typically 1000 microns in diameter. So, about 1 million cloud droplets have to combine to form a single raindrop. We have already discussed the two types of diffusion growthcondensation for a liquid drop, and deposition for an ice crystal. But diffusion growth is too slow to explain what we can observethat precipitation can occur within an hour after a cloud has formed. For a raindrop to form by condensational growth would require more than a couple of days! There must be another mechanism for growing large particles that fall out of a cloud. One way to grow into larger drop would be to combine several smaller particles. To do this the cloud particles have to collide and stick. First, let’s consider water droplets. To get water droplets to collide requires droplets of different sizes because if all the droplets are the same size and are moving together in an updraft with a constant speed, 4-32 the drops will not collide as they are all moving at the same speed. For a collision among droplets to happen requires drops of different sizes, updrafts that vary within the cloud, or a combination of the two. This is similar to cars all moving the same speed on a highway. Different condensation nuclei can generate droplets of different sizes. For example, large salt particles can quickly produce relatively large droplets. Once different sized particles form in the cloud they move at different speeds within the cloud, increasing the chance of a collision. Swirls of wind inside a cloud also can get particles moving in different directions increasing the chance of collisions. Just because two droplets approach one another does not guarantee they will collide. Tiny droplets may flow around large droplets (Figure 4.24). A moving large droplet creates an air current that around it. In a similar way when small cloud droplets approach large drops, they may flow around the drop, because of the air current produced by the large drop. Once a drop reaches a size where the downward force of gravity exceeds the updraft force, it falls downward through the cloud. As the drop falls through the cloud, it sweeps out smaller droplets in its path, collecting them and growing by coalescence. The longer a particle stays in the cloud, the more particles it can collect and the larger it grows. The thicker the cloud the more time a particle can stay in the cloud, colliding and collecting other drops. This is why only clouds with a large vertical extent produce large size precipitation particles. This process of combining particles through collision and coalescence is an important mechanism for forming precipitation in clouds composed of only liquid water droplets. It is an important mechanism for forming precipitation in the Tropics. In other 4-33 regions of the world, clouds contain ice particles, even in the summertime. Other mechanisms for forming precipitation are available when a cloud contains ice. When an ice crystal falls through a cloud it may Accretion is the process of ice also collide with and collect super-cooled water drops. crystal growth by collision and coalescence of water droplets. This process of sweeping-out super-cooled cloud drops is called accretion. When ice crystals collide with super-cooled drops the drop freezes. Accretion is very effective and the particle quickly grows. An ice particle produced by the accretion process that has a size between 1 mm and 5 mm (0.04 to .2 inches) and no discernible crystal habit is called graupel (pl. graupeln). Upon collision and freezing the super-cooled water often traps air bubbles. Because of this trapped air, the density of a graupel is low and can easily be crushed, unlike a hailstone. Accretion can be particularly rapid if there is a lot of super-cooled water in the cloud. When the super-cooled water freezes, latent heat of fusion is added to the riming particle, warming it. But the particle is also losing heat to its environment. If the latent heat added through riming exceeds the heat losses, the accreted water does not completely freeze and a ‘spongy’ ice particle results. Aggregation is the process by which ice crystals collide and form a single ice particle (Figure 4.25). The Aggregation is the process of ice crystal growth by collision and adherence of ice crystals. aggregation effectiveness is based on the shape of the crystals. If two dendrites collide it is likely that their branches will become entwined and the two crystals will stick together. When two plates collide there is a good chance that they will bounce off one another. Temperature also plays a role in aggregation. If the temperature of one crystal is slightly above freezing it maybe encased in a thin film of 4-34 liquid water. If this particle collides with another crystal, the thin film of water may freeze at the point of contact and bond the two particles into one. This is similar to what happens when you remove a tray of ice from the freezer when your fingers are moistthe tray sticks to your fingers. A snowflake is an individual ice crystal or an aggregate of ice crystals. Snowfalls are more commonly composed of snowflakes that are aggregates of ice crystals. Snowflakes composed of aggregates can sometimes reach three or four inches in size. All ice crystals are hexagonal, six-sided, in shape. Since the crystal habit depends on the environment temperature and vapor pressure the crystal traveled in during formation and growth, and the unlikelihood of two crystals experiencing the same environmental conditions during growth, there is some truth to the saying "no two snowflakes are identical". How big a droplet or crystal grows by collision processes depends on how long it stays in the cloud. This length is determined by the strength of the updraft is and the thickness of the cloud. Cumulonimbus clouds have strong updrafts and are thick and so particles can stay in the cloud a long time and grow to a large size. Stratus clouds are shallow in depth and have much weaker updrafts than cumulonimbus. Particles may not stay in a stratus cloud long, and large particles rarely form. The Bergeron-Wegener Process The saturation vapor pressure over a surface of water is different than the saturation vapor pressure over a surface of ice. Let’s go back to our sealed beaker experiment (Figure 4.26). One beaker contains super-cooled water at a temperature of 5C (23F) and the second contains ice at the same temperature. Both beakers are sealed 4-35 with a cover. Eventually, equilibrium is reached between the molecules leaving and entering the air above. The average kinetic energy of the molecules in the super-cooled water, ice and air above these surfaces is the same. Since the bonding forces in ice are much stronger than water, fewer molecules have the energy to escape the ice than the number leaving the water. This means, in equilibrium, there will be less molecules of water in the vapor phase in the beaker with the ice than the beaker containing water. Fewer molecules at the same temperature means the pressure exerted by the molecules is smaller. Thus, the saturation vapor pressure over ice is less than over water. Both the saturation vapor pressure over ice and over water depend on temperature only (Figure 5.27). Consider an ice crystal surrounded by super-cooled droplets (Figure 4.28). If the air is saturated (100% relative humidity) with respect to the water droplets, it will be supersaturated with respect to the ice crystals. So, water vapor molecules will deposit onto the crystal, lowering the relative humidity of the air. In response, the water droplets evaporate supplying water molecules to the air that then deposit on the crystal. Put another way, ice crystals grow at the expense of water droplets. This ice crystal growth process is called the Bergeron-Wegener process because it was first proposed by Alfred Wegener (1880-1930) in 1911 and developed more extensively by Tor Bergeron (18911977). The Bergeron-Wegener growth process requires diffusion and so is limited in how effectively it can produce large particles. Its main importance is as a bridge for the gap between condensational growth, which can effectively grow 10-micron particles and collision growth that requires 40-micron particles. Bridging this gap can turn a nonprecipitating cloud into a precipitating one. 4-36 This ice crystal growth process is fastest when the differences in the saturation vapor pressure between water and ice is large. These differences are largest when the air temperature is between -12C and -17C (1.5F and 10.5F). Different air temperatures have different preferred crystal growth habits and at this temperature regime crystals tend to grow in the dendrite shape. Dendritic crystals grow the fastest by the BergeronWegerner growth process. They are also a frequent crystal shape observed in snow, which forms in this temperature range highlighting the importance of the Bergeron-Wegerner growth process. Rain, freezing rain, sleet, snow and hail What happens when a particle falls out the base of a cloud? In some cases it may never reach the ground. For instance, since the air below the cloud base is not saturated (relative humidity is less than 100%) all falling precipitation particles begin to evaporate. If the atmosphere below the cloud is very dry, the particle will evaporate before reaching the ground. Virga is rain that evaporates before reaching the surface (Figure 4.29). (Box 4.2 lists the different precipitation types). Ice crystals may also reach large sizes and fall out of a cloud. These may also be carried horizontally by the strong winds aloft and evaporate in dry atmospheres. These evaporating ice particles are referred to as fall streaks. Sometimes particles actually do reach the surface as rain, snow, freezing rain or sleet. The type of precipitation depends on the temperature structure below the cloud bases. For the last three the temperature must be below freezing. In the midlatitudes, where cloud top extend to altitudes where the temperature is below freezing, it is likely that the rain that reaches the surface was at one time a frozen particle. 4-37 Figure 4.30 is an example of the type of weather conditions that lead to snow, sleet, freezing rain, and rain. These conditions often occur with the passage of a warm front. In all these weather conditions the precipitation particles were at one time an ice particle. The thick lines represent the vertical profile of temperature for a given region. The dashed line represents the melting line, the altitude at which the temperatures are 0C (32F). Far in front of the warm front the temperature throughout the atmosphere is below freezing. The crystals, or aggregate of crystals, never melt and the Snow is precipitation in the form of crystals or aggregates of crystals. Sleet is frozen raindrops. Freezing rain is super-cooled rain that freezes on contact with objects. region gets snow. If graupeln falls from the cloud, they may reach the ground as round, soft clumps of ice called snow pellets. Sleet, or frozen raindrops, occurs when a surface inversion is present. Snowflakes fall below the melting line and become raindrops. But before reaching the surface the rain falls into another layer of the atmosphere below freezing and freezes into translucent balls of ice called sleet. When sleet hits the surface, it bounces, and does not cover objects with a sheet of ice. The difference between sleet and freezing rain is the depth of the surface inversion. If the inversion is not strong, the raindrops fall into the freezing layer of the atmosphere and cool but do not freeze. The rain reaches the ground as super-cooled drops that freeze when they come into contact with objects. Freezing rain covers everything in a sheet of ice generating extreme traffic hazards and damage to tress and power lines (Figure 4.31). Freezing rain often produces a beautiful sight. As a rule of thumb, freezing rain occurs when the surface temperature is between 0C (32F) and -3C (27F) while sleet occurs when the surface temperature is colder than -3C and an inversion exists. 4-38 Rain occurs when the temperature is above freezing near the ground and the melted particles reach the ground as liquid drops. Rain showers refer to rain that starts, changes intensity, and stops suddenly. The difference between drizzle and rain is size of the drops. Drizzle are liquid drops with a diameter of less Rain intensity is classified as light (0.5 mm of water per hour), moderate (0.4 to 4 mm per hour) or heavy (greater than 4 mm per hour). Snow intensity is recorded as light (less than 0.5 cm of snow per hour), moderate (0.4 to 4 cm per hour) or heavy (greater than 4 cm per hour). Accumulation rates of snow are not measured in drifts. than 0.5 mm (0.02 inches). If the drizzle particles freeze before reaching the ground, they are called snow grains. Hail is precipitation in the form of large balls or lumps of ice. Hailstones are the individual clumps of ice (Figure 4.32). Hail is produced in cumulonimbus clouds that have strong updrafts. Hailstones begin as aggregates of ice crystals that fall through the cloud. During descent the ice pellets may encounter a strong updraft and be carried upward. As the updraft weakens the particle falls down through the cloud growing by accretion, aggregation, and deposition. The growing particle may again encounter another strong updraft as it falls and be carried to high altitudes in the cloud again. In this manner the hailstone is cycled through the storm. The size of a hailstone is determined by the number of times it is cycled through the storm. The record hailstone was found in Coffeyville, Kansas and weighs 766 grams (1.67 pounds) and has a diameter of 14 centimeters (5.5 inches). Layers of ice build on a hailstone as it cycles through the storm. The layers appear as either clear ice or white ice. When the hailstone collides with large drops of water, drops spread over the hailstone before freezing, leaving a clear layer of ice. White ice forms when the hailstone collects small super-cooled drops. The small droplets quickly 4-39 cool and trap air bubbles in the ice. The reason trapped air bubbles make ice look white is a topic of Chapter 6. Rime also covers objects in ice, though rime is much different from sleet, snow or freezing rain. Rime forms when a super-cooled fog is present and surface temperatures are below freezing (Figure 4.33). In these conditions, surfaces such as pine needles and grass act as ice nuclei. Super-cooled fog drops freeze on contact with these objects. Rime also grows on the surfaces by the Bergeron-Wegener process, fog droplets evaporate and the water molecules deposit on the surface. 4-40 Putting it all together Let's investigate how concepts in this chapter integrate with concepts presented in previous chapters by analyzing airflow over a mountain. In doing this we want to form a consistent view that explains the observation that semi-arid regions are often found downwind, or the lee side of a large mountain range. Let's begin with air blowing on a large mountain range oriented in the NorthSouth direction (Figure 4.34). The mountain range is 3 km in altitude and the air has an initial temperature 10C with a dewpoint temperature of 0C. An air parcel incident on the mountain is lifted. As the parcel rises it expands and cools at the adiabatic lapse rate of 10C for each kilometer (Chapter 2). As the temperature decreases the relative humidity increases as the temperature approaches the dew point temperature. Eventually, the temperature will equal the dew point temperature and a cloud will form at the lifting condensation level, marking the cloud base. As the air continues to rise water vapor continually condenses to form cloud droplets. A phase change of water vapor to a liquid releases energy, warming the parcel through latent heating, which causes the parcel to cool at the moist adiabatic lapse rate of approximately 6C for each kilometer (Chapter 2). As the parcel continues to be orographically lifted, cloud droplets will grow by condensation and by collision and coalescence. As the moist parcel continues to rise, it continues to cool due to expansion. Eventually, some of the liquid drops freeze and the cloud becomes glaciated. The cloud particles can then quickly grow by the Bergeron-Wegener process, accretion and aggregation. The particles continue to grow and eventually they fall to the ground as snow. Water molecules leave the air parcel with the snowfall. The lost latent heat cannot 4-41 be used to warm the parcel as the water molecules are now on the ground, making good ski conditions. At the top of the mountain, the relative humidity is 100%, the parcel temperature is -12C, and the air begins to flow down the leeward slope of the mountain. As the air sinks, it warms and thus the relative humidity decreases. The cloud particles evaporate as the air sinks and warms. With no cloud in the parcel, the parcel now warms at the dry adiabatic lapse rate. When the parcel returns to the base of the mountain its temperature is now 18C. In addition to being 8C warmer than when it started, there are fewer water molecules in the parcel because of the precipitation on the windward side. Increasing the temperature and removing water vapor from the air lowers the relative humidity. This is the reason why regions located on the leeward side of a mountain range, are so warmer and drier than their windward counterparts. Providing that the prevailing wind (Chapter 1) is perpendicular to the mountain range, the windward side will have more cloud cover than the leeward side. The increased cloud amount will likely reduce the annual temperature range (Chapter 3) on the windward side compared to the leeward side. This is supported by weather observations on each side of the Cascade Mountains (Table 4.2). Table 4.2 Difference in temperature, cloud cover and precipitation on the windward and lee side of the Cascade Mountains (Source: Landsberg) Windward Side (Tacoma) Lee Side (Yakima) 41 31 Mean Winter Temp F 62 69 Mean summer Temperature F 25 45 Mean Annual Range F Number of cloudy days per year 292 252 Average Annual Precipitation 38.7 7.2. (in) 4-42 Summary The hydrological cycle describes the water budget of the Earth. Water in the atmosphere can be found in the form of water vapor, clouds, and precipitation. The relative humidity, mixing ratio, dew point temperature, and vapor pressure are different methods of representing the amount of water vapor in the atmosphere. A cloud is a suspension of water droplets or ice crystals. The formation of a cloud requires water vapor, saturation, and nucleation. There are two general methods of nucleation, or particle formation, heterogeneous or homogeneous. Heterogeneous nucleation involves aerosols while homogeneous nucleation involves only water molecules. Heterogeneous nucleation occurs on nuclei called cloud condensation nuclei or ice nuclei. Most clouds are formed when air cools to high humidities when it is lifted. The four primary mechanisms for lifting air are orographic lifting, frontal lifting, convection, and convergence. Observations of cloud type and characteristics of precipitation can provide much information about the state of the atmosphere. The five basic families of clouds are low, middle, high, those with extensive vertical development, and those that are precipitating. The ten basic cloud types are cirrus, cirrostratus, cirrocumulus, altostratus, altocumulus, cumulus, stratus, stratocumulus, nimbostratus, and cumulonimbus. A fog is a suspension of minute water droplets in the atmosphere near the ground. Fogs form when air becomes saturated due to cooling and/or. Fogs form either through cooling of the air to the dew point (producing radiation fog, advection fog, or up-slope fog) or by increasing the dew point through the addition of water vapor molecules to the air (producing steam fog or evaporation fog). 4-43 The precipitation process requires that cloud droplets or ice particles grow large enough for gravity to overcome the cloud updrafts. Growth by diffusion of water vapor (i.e., condensation and deposition) is important in the formation of cloud particles. The time required to form a raindrop by diffusional growth is too long to explain why precipitation is observed to form in less than one hour. The two primary methods of forming precipitation-sized particles are collision and coalescence and the ice crystal process. Collision and coalescence is the growth process involving water drops that collide and form one drop. Growth processes that involve ice crystals are the BergeronWegener process, accretion, and aggregation. The Bergeron-Wegener process is the growth of an ice particle that results because of the saturation vapor pressure differences between ice and water at temperatures below freezing. Accretion is the collection of super-cooled water drops by crystals while aggregation is the mutual collection of crystals. The most common forms of precipitation are drizzle, rain, rain showers, freezing rain, sleet, snow, and hail. Drizzle is different from rain in that it is composed of very small droplets. Freezing rain and sleet form when there is a temperature inversion near the surface. 4-44 Terminology You should understand all of the following terms. Use the glossary and this Chapter to improve your understanding of these terms. Accretion Freezing Rain Aggregation Frost Altocumulus Hail Altostratus Heterogeneous nucleation Bergeron-Wegener Process Homogeneous nucleation Cirrus Ice crystals Cirrocumulus Ice nuclei Cirrostratus Lifted condensation level Cloud droplet Nimbostratus Crystal habit Nucleation Collision and coalescence Mixing ratio Condensation nuclei Precipitation Cumulus Rain Cumulonimbus Rain shower Curvature effect Relative humidity Dew Saturation vapor pressure Dew point temperature Sleet Drizzle Snow Evaporation Snowflake Fog Solute effect 4-2 Stratus Supersaturation Vapor pressure 4-2 Review Question 1. Sometimes a fog will appear over a roadway after a summer rain shower. What type of fog would this be? 2. Why are pressure cookers sometimes used to cook food at high altitudes? 3. Explain while advection would cause maritime stations on the east end of a continent to have a maximum occurrence of fog in summer and a minimum occurrence in the winter. 4. Clouds over land have approximately 500 million to 1 billion cloud droplets be cubic meter of air. Maritime clouds, ones that form over oceans, typically only have 50 to 100 million droplets per cubic meter of air. Why is the number of cloud droplets so much greater for continental clouds than maritime clouds? 5. Tor Bergeron-Wegener observed that if a fog formed in a forest and the temperature was above 0C (32F) the fog extended down to the ground. If the temperature was below -5C (23F) the fog would not reach the forest floor. Explain his observation. 6. An ice crystal grows for five minutes in a supersaturated environment with a temperature of -1C. The crystal is carried to a different part of the cloud where the temperature is -14C and the environment is still supersaturated. The crystal stays in this region of the cloud for another 5 minutes. Using Table 5.3, draw a picture of what the ice crystal might look like. 7. Visit an art museum and catalogue the type of clouds used in the exhibited paintings. 8. The monthly mean precipitation for Barrow Al are available on the Web. Plot and discuss the annual precipitation cycle of this city. 4-3 9. Put a piece of glass on the roof of your car on a night when frost is predicted and observe where frost forms, and does not form. Explain your observations. 10. Many public restrooms have automatic hand-dryers. The instructions say to place your hands in the airflow and gently rub them together. Explain how this dries your hands. 11. Can freezing rain occur when the ground temperature is 0C (32F)? 12. List the unusual characteristics of water. 13. Explain the meaning of the term “saturation”. 14. List and describe the different types of nucleation. 15. Why is the saturation vapor pressure greater over a curved surface than over a flat surface? 16. What factors determine the size and shape of an ice crystal? 17. Explain why the saturation vapor pressure over ice is different than the saturation vapor pressure over liquid water. 18. Can you dry a wet laundry hung out in freezing weather? Explain your conclusion. 19. What are the differences and similarities between evaporation and boiling? 20. Explain why raindrops do not get bigger than approximately 0.5 cm. 21. List and describe the different types of fog. 22. List and describe the 10 major cloud types. 23. What factors determine the growth of a cloud droplet? 24. What factors determine the growth of an ice crystal? 25. Explain what happens when super-cooled droplets coexist-exit with ice crystals in a volume of a cloud. 26. Describe the differences between rain, freezing rain, sleet and snow. 4-4 Web Activities Current humidity and precipitation maps -- updated daily The thermodynamic diagram revisited How meteorologists draw clouds Animation of collision and coalescence Animation of Bergeron process Relationship between temperature and humidity Satellite loop of fog dissipation Photographs of cloud Identifying cloud types Practice multiple choice exams Practice true/false exam 4-5 Box 4.1 Humidity and Your Health Over 500 deaths in the central United States were attributed to the July 13-14, 1995 heat wave. These deaths did not result from the 100-degree temperatures alone; the amount of water vapor in the atmosphere played a devastating role. When our bodies get hot we cool by sweating. It is not the sweating that cools our bodies but rather the evaporation of the sweat. If the air has a high vapor pressure then the rate of evaporation is reduced, and our body’s ability to maintain a nearly constant, internal body temperature is hampered. This is why we are uncomfortable on hot, muggy days. Very high temperatures in association with high humidities can be dangerous. The Apparent Temperature Index or the Heat Index indicates how hot it feels. It is expressed as a function of air temperature and the relative humidity. It was developed by R. G. Steadman in 1979. The National Weather Service has been reporting this index since 1984. It is reproduced below. Large differences in the vapor pressure of the air and our skin (the surface that is evaporating the sweat) promotes evaporation and therefore makes us feel cooler, as the apparent temperature is less than the actual temperature. The apparent temperature index reflects this fact. In the winter, many houses in northern regions have a very low relative humidity. This results from cold air that is heated. Heating the air decreases the relative humidity. As a result, on cold winter days, the relative humidity in a house can be less than the relative humidity of the Sahara desert! If your thermostat is set at 70F and the relative humidity is only 10%, the apparent temperature is 65F! To feel warmer you can turn up the heat, which continues to lower the relative humidity, or increase the apparent 4-6 temperature by adding water vapor. The moister air will also keep you skin and plants from drying out by reducing evaporation. The apparent temperature index, and the wind-chill equivalent temperature, should be considered as a guideline. Our comfort is determined by other factors than these tables. For example, on a hot humid day a breeze can also make use feel more comfortable by promoting evaporation and by transporting heat away from our bodies. 4-7 The Apparent Temperature Index Relative Humidity Air Temperature (F) (%)% 70 75 80 85 90 95 100 105 110 115 120 0 64 69 73 78 83 87 91 95 99 103 107 10 65 70 75 80 85 90 95 100 105 111 116 20 66 72 77 82 87 93 99 105 112 120 130 30 67 73 78 84 90 96 104 113 123 135 148 40 68 74 79 86 93 101 110 123 137 151 50 69 75 81 88 96 107 120 135 150 60 70 76 82 90 100 114 132 149 70 70 77 85 93 106 124 144 80 71 78 86 97 113 136 90 71 79 88 102 122 100 72 80 91 108 Red: Great risk to health, heat stoke or sunstroke imminent. Magenta: Risk of sunstroke, heat exhaustion or heat cramps. Yellow: Prolonged exposure and physical activity could lead to sunstroke, heat exhaustion or heat cramps. Cyan: Prolonged exposure and physical activity may lead to fatigue. 4-8 Box 4.2 Precipitation Types Formation of a precipitation Precipitation particle is a complex process that Droplet or ice crystal growth requires several events, or steps, before Condensation or deposition occurring. You can think of the main Saturation steps as rungs on the “precipitation Ascent and cooling ladder” shown in the accompanying Moist air with nuclei figure. Different precipitation types are listed in the accompanying table. 4-9 Hydrometeor Description Drizzle Liquid water drops less than 0.5 millimeters (0.02 inches) in diameter Rain Drops with diameters greater than 0.5 millimeters (0.02 inches). Also widely scatter drops of smaller size. Freezing Drizzle Drizzle that freezes on contact with the ground Freezing Rain Rain that freezes on contact with the ground. Snow Precipitation in the form of ice crystals. They can be of various sizes. Snowflakes are aggregates of ice crystals, often of the dendritic form, and can reaches several inches in size. Snow pellet White, opaque grains of ice with diameters between about 2-5 millimeters or graupel (0.1 to .2 inches). Can be spherical or conical. Snow pellets are brittle. Snow grain Small, white opaque grains of ice. Sizes are generally less than 1 millimeter (.0.4 inches). The ice equivalent of drizzle, they are fairly flat or elongated. Ice pellet Transparent or translucent pellets of ice that are spherical or irregular in shape, but rarely conical. Diameters are less than 5 millimeters (0.2 inches). Ice pellets bounce when the hit ground. Sleet is composed of ice pellets. Hail Precipitation in the form of pieces of ice with diameters greater than 5 millimeters (0.2 inches). Hailstones are either transparent, or have concentric layers of transparent and opaque ice. Observed during intense 4-10 thunderstorms. 4-11