Chemistry Test: Naming Compounds and Balancing Equations
advertisement
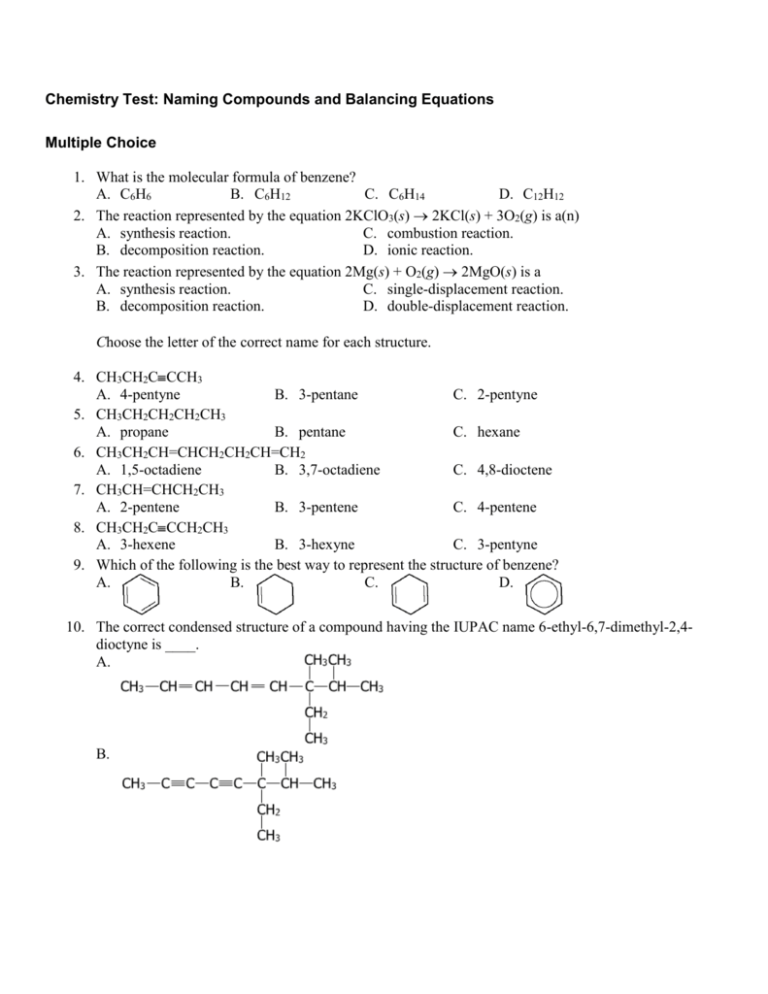
Chemistry Test: Naming Compounds and Balancing Equations Multiple Choice 1. What is the molecular formula of benzene? A. C6H6 B. C6H12 C. C6H14 D. C12H12 2. The reaction represented by the equation 2KClO3(s) 2KCl(s) + 3O2(g) is a(n) A. synthesis reaction. C. combustion reaction. B. decomposition reaction. D. ionic reaction. 3. The reaction represented by the equation 2Mg(s) + O2(g) 2MgO(s) is a A. synthesis reaction. C. single-displacement reaction. B. decomposition reaction. D. double-displacement reaction. Choose the letter of the correct name for each structure. 4. CH3CH2C CCH3 A. 4-pentyne B. 3-pentane C. 2-pentyne 5. CH3CH2CH2CH2CH3 A. propane B. pentane C. hexane 6. CH3CH2CH=CHCH2CH2CH=CH2 A. 1,5-octadiene B. 3,7-octadiene C. 4,8-dioctene 7. CH3CH=CHCH2CH3 A. 2-pentene B. 3-pentene C. 4-pentene 8. CH3CH2C CCH2CH3 A. 3-hexene B. 3-hexyne C. 3-pentyne 9. Which of the following is the best way to represent the structure of benzene? A. B. C. D. 10. The correct condensed structure of a compound having the IUPAC name 6-ethyl-6,7-dimethyl-2,4dioctyne is ____. A. B. C. D. 11. Sulfur dioxide, oxygen, and water combine to produce sulfuric acid (2 SO2 + O2 + 2 H2O 2 H2SO4). This chemical reaction is A. an example of combustion. B. a synthesis reaction. C. a replacement reaction. D. a decomposition reaction. 12. Which of the following groups is characteristic of an alcohol? A. -CO B. -COOH C. -NH2 D. -OH 13. Which equation is not balanced? A. 2H2 + O2 2H2O B. 4H2 + 2O2 4H2O C. H2 + H2 + O2 H2O + H2O D. 2H2 + O2 H2O 14. The reaction represented by the equation Mg(s) + 2HCl(aq) H2(g) + MgCl2(aq) is a A. composition reaction. C. single-displacement reaction. B. decomposition reaction. D. double-displacement reaction. 15. When the equation Fe3O4 + Al Al2O3 + Fe is correctly balanced, what is the coefficient of Fe? A. 3 B. 4 C. 6 D. 9 16. Which coefficients correctly balance the formula equation NH4NO2(s) N2(g) + H2O(l)? A. 1, 2, 2 B. 1, 1, 2 C. 2, 1, 1 D. 2, 2, 2 17. Which group of organic compounds can be considered derivatives of ammonia, NH3? A. ester B. ketone C. aldehyde D. amine 18. The structure of 3-ethyl-5,7-dimethyl-5-propylnonane is ____. A. C. B. D. 19. Which of the following is a balanced chemical equation? A. H2O2 H2O + O2 B. 2 Fe2O3 + 3 C 4 Fe + 3 CO2 C. SO2 + O2 + 2 H2O 4 H2SO4 D. 2 Mg + HCl MgCl2 + H2 20. The correct structural formula of 1,2-diethyl-2,3-dimethyl-6-propylcyclooctane is ____. A. C. B. D. 21. Name the cycloalkane given below. A. 1,2,4-trimethylcyclohexane C. 1,2,4-trimethylcyclopentane B. 1,2,4-dimethylcyclopentane D. 1,3,5-trimethylcyclopentane 22. The reaction represented by the equation Pb(NO3)2(aq) + 2KI(aq) PbI2(s) + 2KNO3(aq) is a A. double-displacement reaction. C. decomposition reaction. B. synthesis reaction. D. combustion reaction. 23. The reaction represented by the equation 2HgO(s) 2Hg(l) + O2(g) is a(n) A. single-displacement reaction. C. combustion reaction. B. synthesis reaction. D. decomposition reaction. 24. Which coefficients correctly balance the formula equation CaO + H2O Ca(OH)2? A. 2, 1, 2 B. 1, 2, 3 C. 1, 2, 1 D. 1, 1, 1 25. The correct IUPAC name of the compound is ____. A. 3,4-dimethylpentane C. 2,3-dimethylpentane 26. 27. 28. 29. 30. 31. B. 2,3-dimethylbutane D. 2,2,3-dimethylbutane What is the balanced equation when aluminum reacts with copper(II) sulfate? A. Al + Cu2S Al2S + Cu B. 2Al + 3CuSO4 Al2(SO4)3 + 3Cu C. Al + CuSO4 AlSO4 + Cu D. 2Al + Cu2SO4 Al2SO4 + 2Cu What is the general formula for ethers? A. R–O–R' B. R–COOH C. R–COO–R' D. R–CHO The reaction represented by the equation Cl2(g) + 2KBr(aq) 2KCl(aq) + Br2(l) is a(n) A. synthesis reaction. C. single-displacement reaction. B. decomposition reaction. D. combustion reaction. Which of the following suffixes is used in naming alcohols? A. -al B. -oic C. -ol D. -ane In an equation, the symbol for a substance in water solution is followed by A. (1). B. (g). C. (aq). D. (s). Which of the following is a balanced chemical equation? A. AgNO3 + NaCl 4AgCl + 2NaNO3 B. 2AgNO3 + 2NaCl 3AgCl + 2NaNO3 C. AgNO3 + NaCl AgCl + NaNO3 D. AgNO3 + 2NaCl AgCl + 3NaNO3