Types of Chemical Reactions
advertisement

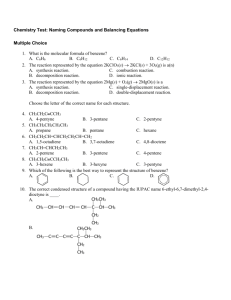
Types of Chemical Reactions 1. Synthesis Reactions • A synthesis reaction occurs when two or more simple substances combine to produce a more complex substance. • AKA: Combination reaction. • The general equation for a synthesis is: A + B AB • HINT: If there is only one product – it is likely a synthesis. Examples of Synthesis Reactions • CO2 + H2O H2CO3 • 4Fe + 3O2 2Fe2O3 • Li2O + H2O 2LiOH 2. Decomposition Reactions • A decomposition reaction occurs when a complex substance is broken down into two or more simpler substances. • Heat is often used to aid in decomposition reactions – these reactions that employ heat are called thermal decompositions. • Decompositions and synthesis reactions are opposites. • The general equation for a decomposition reaction is: AB A + B • HINT: If there is only one reactant – it is likely a decomposition reaction. Examples of Decomposition Reactions: • NH4NO3 N2O + 2H2O • Ca(OH)2 CaO + H2O • 2H2O2 2H2O + O2 3. Single Displacements • A single displacement reaction occurs when a single element takes the place of one of the elements in a compound. • AKA: Single Replacement • The general equation for a single displacement reaction is: AB + Z ZB + A • Metals displace metals while nonmetals displace nonmetals. • HINT: The single mysterious loner moves into town and breaks up the happy couple! Examples of Single Displacement Reactions • Fe + CuSO4 FeSO4 + Cu • 2K + MgO K2O + Mg • 2CuF + Ba BaF2 + 2Cu Not So Fast There… • The lone element doesn’t always break up the couple! We can use a tool called the activity series to predict if the compound will stay together or break up. • The activity series is a list of metals and hydrogen that are arranged in order of reactivity. Li K Ba Ca Na Mg Al Zn Fe Ni Sn Pb H Cu Hg Ag Au • The rule is that you can only be displaced by an element that is to the left of you. This makes Lithium the strongest and Gold the weakest. • There is also a halogen activity series – it is used to predict reactions with halides. F Cl Br I Using the Activity Series You can use the activity series in three ways: 1) 2) 3) • Straight Forward Single Displacements – • Use the rule of “whoever is more to the left wins” to see if there is a reaction or not. Reactions with Acids – • Straight forward Single Displacements Reactions with Acids Reactions with Water Acids contain hydrogen (positive like the metals). If you are to the left of hydrogen – you react and take its place – if you are to the right – there is no reaction. Reactions with Water – Only the first five elements (Li K Ba Ca Na) will react with water. It will form a hydroxide and hydrogen gas. 4. Double Displacements • A double displacement reaction always involves two ionic compounds that switch partners with each other. • Again, positive ions switch with positive ions (and/or vice-versa). • The general equation for a double displacement reaction is: AB + XY AY + XB HINT: Two couples switch partners at the dance. Examples of Double Displacement Reactions: • Pb(NO3)2 + 2KI PbI2 + 2KNO3 • Na2SO3 + 2HCl 2NaCl + H2SO3 • 2NaOH + H2SO4 2H2O + Na2SO4 Not So Fast There…Again! • There are three outcomes for a double displacement reaction: 1) Precipitate – solid formed from two liquids. • Use the solubility rules. 2) Gas – some compounds form products that break down further into gases. 3) Water – results from a neutralization between an acid and a base. 5. Combustion Reaction • A combustion reaction occurs when a substance (the “fuel”) reacts very rapidly with oxygen to form carbon dioxide and water. • Combustion reactions release a good deal of energy in a very short period of time. • The general equation for a combustion reaction is: Fuel + O2 CO2 + H2O • HINT: Something combines with oxygen to produce carbon dioxide and water. Incomplete Combustion • If a combustion occurs at a lower temperature, it may result in an incomplete combustion. • The products of an incomplete combustion are water, carbon dioxide, carbon monoxide and carbon (a solid residue). • The general equation is: Fuel + O2 H2O + CO2 + CO + C THE END