Homework 4
advertisement

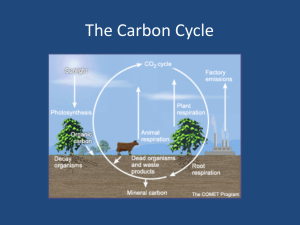
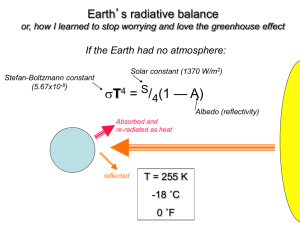
Homework #4 ATMS 449 March 8, 2005 Due March 29, 2005 1. If wood has a density of 0.8 g/cm3 and a carbon content of 50% by mass, what is the size of a cube of wood that would contain 1 GtC (1015 g of carbon)? What does this tell you about the amount of carbon stored in a forest? 2. Explain why the chemical weathering of silicate rocks results in a stabilizing effect on Earth’s climate. 3. Anthropogenic climate change a. At present the atmosphere contains roughly 760 Gt(C) (billion metric tons of carbon in the form of CO2, thus the atmospheric CO2 concentration is about 380 ppm. Total recoverable fossil fuel reserves contain at least 4200 Gt(C), mostly in coal. Currently about half the CO2 produced by the burning of fossil fuels remains in the atmosphere, and the other half is taken up by the terrestrial biosphere or dissolves in the oceans. If this ratio remained the same and we burned all the fossil fuels instantaneously, what would the atmospheric CO2 content (in both Gt(C) and in ppm) then be? b. Climate models predict that each doubling of atmospheric CO2 concentration will cause the mean global surface temperature to increase by roughly 2 to 5°C. Use the ratio of the new CO2 amount in Gt(C) to the current level of 760 Gt(C) to estimate how much the mean global surface temperature would warm. c. The actual problem of global warming may be more serious than the above calculation. Forests and soils worldwide together contain a total of 2100 Gt(C) that could theoretically end up in the atmosphere. Plus, as the ocean absorbs more CO2, it becomes more acidic and less able to continue absorbing CO2. Now suppose we burn all the fossil fuel reserves and also through deforestation and changing land use cause one-third of the carbon stored in forests and soils to be released into the atmosphere, but assume all of this carbon stayed in the atmosphere and none was removed by dissolving into the ocean or by CO2 fertilization. What would the atmospheric CO2 concentration be in this case? How much warming would this increase in CO2 cause? 4. Fossil fuel use and deforestation are cited as the primary reasons for the increasing atmospheric CO2 level. Someone might suggest that simple breathing by the growing human population is raising the atmospheric CO2 level. a. The average American diet of 2400 Calories per day translates into energy use of 116 W (or J/sec), which equals approximately 3.6 billion Joules (3.6 GJ) of energy use per year. Assume the CO2 emission factor for food is 30 kg(C)/GJ (compared to 25 for coal, 20 for oil, and 15 for natural gas). How many kg of carbon per year does the average American exhale? How does this value compare to the total emissions per American of 6 tons of carbon per year? b. Assuming all 6 billion people on Earth released the same amount of carbon in respiration as an average American, how many Gt(C) per year would the world’s population exhale? How does this amount compare to the approximately 7 Gt(C) added each year by fossil fuel use? Also, how does this amount compare to the annual cycle of 60 Gt(C) involved in plant photosynthesis and respiration/decay? 5. Atmospheric carbon dioxide concentration a. During which season is the rate of photosynthesis greatest relative to the combined rate of respiration/decay, and in which season is it smallest? Explain your answers. b. At what times of the year is the atmosphere in steady state with respect to CO2 (the release into the atmosphere equals the amount taken out)? c. Using the answers for parts (a) and (b), sketch a graph depicting how the rate of net photosynthesis varies with the seasons. Also sketch a graph showing how the rate of net respiration/decay varies with the seasons. Draw each graph extending over at least three years, and clearly label both axes of each graph. d. For the graphs in part (c), estimate the maximum net rates of CO2 removal and addition during those seasons when these changes are greatest.