Inorganic Nitrogen and N Mineralization
advertisement

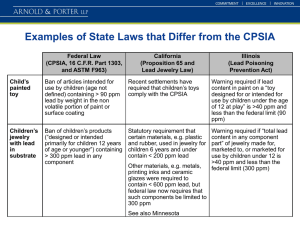
Gill Ecosystem and Global Change Ecology Lab Protocol: Inorganic Nitrogen, Net N mineralization, and Net Nitrification Created by: Lafe Conner (with input from Debbie Rigby’s protocol) Edited by: Date Last Modified: 6 February 2016 Soil Sampling Protocol for Inorganic N and net N Mineralization Items needed: Soil gouge auger (small diameter silver colored) Soil auger with compact slide hammer (red and black 5 cm diameter) Clear plastic tube liners that fit in the slide hammer auger Large zip-lock storage bags Sharpie permanent marker Cooler with ice packs 1. Mark gouge auger with a piece of tape at 15cm (same depth as the plastic tubes) 2. Place a clear plastic tube liner in the 5cm auger with the slide hammer 3. Pound this into the ground up to the depth of the tube 4. Remove the clear tube from the auger 5. Place the clear tube with soil in it back in the hole 6. Take 3 soil cores 15 cm deep with the gouge auger and place these into the zip-lock bag 7. Label the bag with the site information, the date, and mark it as “initial” 8. Place the soil sample in the cooler as soon as possible and keep it cool 9. Leave the clear tube in the soil at the site for 28 days then retrieve it 10. Bring the samples back to the lab and process them within 24 hours 11. Keep samples in the refrigerator before extraction 12. After extraction place the liquid extract into the freezer and leave the bags of soil open so they can dry (if you plan to use these sample for soil texture analysis or to measure matrix potential) Soil inorganic N:1 Sample preparation for NO3 and NH4 Use also for Net Nitrogen Mineralization and Net Nitrification 2 Items needed: 125 ml Erlenmeyer flasks 50 ml centrifuge tubes 50 ml syringe Parafilm 0.5 M K2SO4 -Dissolve 87.13 g in 1 liter double DI water 1. 2. 3. 4. 5. 6. Tare balance with a 125 mL Erlenmeyer flask Label the flask with the sample ID, use permanent marker Weigh 15 g (+/-0.5 g) of soil into the flask Record the exact mass of the soil on the data sheet and the electronic spreadsheet Weigh an empty soil tin Record the number (label) and mass of the tin on the data sheet and the electronic spreadsheet 7. Weigh 4 g (+/-0.5 g) of soil into a soil tin 8. Record the mass of the soil in the tin on the datasheet and the electronic spreadsheet 9. Weigh approximately 12-18 samples 10. Dispense 30 mL of 0.5 M K2SO4 into tube and cap the tube (1:2 weight to volume ratio of 0.5 M K2SO4, see reagent recipe above for 0.5 M K2SO4) 11. Shake for 60 minutes on shaker at low speed (100 rpm) 12. While the samples are shaking weigh the remaining samples and prepare the funnels and filters to filter the samples 13. Use the funnels and Whatman filter paper (44, 11 or 12.5 cm diameter) 14. Place the filters in the funnels above the labeled centrifuge tubes (label tubes with the experiment name, “N” for nitrogen, the date, and the Sample ID). Use permanent marker to label the tubes. 15. Pour the supernatant into the filter and let stand for approximately 1 hour until the liquid has filtered through to the centrifuge tube 16. Cap the centrifuge tubes and place them into the freezer 17. Place the soil tins into the drying oven for 48 hours on low 18. Reweigh the tins and record their dry mass on the data sheet and the electronic spreadsheet 19. store at -20C until the samples are analyzed 20. Wash the dishes using soap, rinse three times with regular water and three times with DI water 21. Use acetone to remove the marker (use ventilation hoods while working with acetone) Ammonium, NH4+ 1 2 The original protocol for the lab work was written by Debbie Rigby and modified by Lafe Conner. If using this procedure for net N mineralization you will take two soil cores in the field. Bring one back to the lab and analyze it (initial), but leave the other in the field for 28 days (incubated). For net N mineralization sum the NH4+ and NO3- values from the initial core and subtract this value from the sum of NH 4+ and NO3- from the incubated core. You can also calculate net nitrification by subtracting the initial NO3- value from the incubated NO3value. Preparing reagents3 Equipment and Chemical list: 100 ml volumetric 100 ml container with cap Weigh paper Parafilm Gloves Nanopure water Sodium nitroprusside (sodium nitroferricyanide) (Na2[Fe(CN)5NO]·2H2O) Sodium salicylate (C7H5NaO3 ) Sodium citrate (C6H5Na3O7 ) Sodium tartrate (C4H4Na2O6) Sodium hydroxide (NaOH) Bleach Reagent A: 1. Fill a 100 ml volumetric 1/2 full with nanopure water 2. Pre-weigh and add the following: 0.05 g sodium nitroprusside (sodium nitroferricyanide) 13 g sodium salicylate 10 g sodium citrate 10 g sodium tartrate 3. Cap the top with parafilm and shake until all the chemicals are dissolved 4. Fill the volumetric to the meniscus with a water bottle labeled “nanopure water” 5. Pour the liquid into an new container and label it “Reagent A” Warnings: 33 These notes are from Megan Taylor who ran this procedure for the 2012 samples. “For this procedure I made reagents A and B. I stored reagent A in a container covered in foil to block out any light. Both reagents were stored in a refrigerator for up to 3 weeks. I put the sample liquid into microcentrifuge tubes and would use a microcentrifuge to make the white precipitate go to the bottom of the tube before extracting the sample. I created a 6 ppm standard. I would put 430ul K2SO4 into 6 microcentrifuge tubes. Then I would mix in the 6 ppm. Then take the mixture from that tube and put it into the next tube and so on. This created standards with values of 3, 1.5, .75, .375, .1875, and 0 ppm. When putting the samples into the plates, I would put 200ul of the samples first. Then add 50ul of reagent A and 50ul of reagent B. Then I would wait 1 hour and use the spectrophotometer to read the samples. I created a graph from the standard ppm and abs values. I then used the regression equation to convert the absorbance values of the samples to ppm. Samples a blue color when read” For samples with NH4+ concentrations higher than 3 ppm, discovered after the first run, we ran them a second time after diluting the sample 1:1 with 0.5 M K2SO4 (diluted by ½). If the diluted sample was still higher than 3 ppm we diluted it 1:2 with 0.5 M K2SO4 (diluted by 1/3). Nanopure water is the clean laboratory water that is used in molecular and analytical chemistry. If you think it is contaminated or the amount in the nalgene is low please refill it with more nanopure from the Genetics Molecular Lab When weighing chemicals make sure you tare the balance and wear gloves Reagent A is light sensitive, so wrap aluminum foil around the bottle for storage Reagent B: 1. Weigh 6 g sodium hydroxide (NaOH) 2. Using a 100 ml container with cap, dissolved the 6 g NaOH in 100 ml water 3. Add 2 ml bleach with a pipette or graduated cylinder Reagent A and B can be stored for several weeks in the refrigerator Creating an NH4+ standard and standard curve Making a 100 ppm stock solution Equipment and chemical list: 500 ml volumetric Ammonium sulfate ((NH4)2SO4) Parafilm 1. 2. 3. 4. Fill a 500 ml volumetric 1/2 full with nanopure water Add 0.236 g of (NH4)2SO4 Cap the top with parafilm and shake until all the chemicals are dissolved Label and store in a 500 ml glass bottle This is for a 100 ppm stock solution that is used to generate you standard curve Warning: When creating the standards and analyzing samples wear gloves Making a standard curve for soils with NH4+ concentrations up to 3 ppm. This is the standard curve that we use for most wildland soils since inorganic N does not usually hang around in this form. The standard curve includes 6 standards: 3, 1.5, 0.75, 0.375, 0.1875, and 0. To calculate the standards we use the following equation: C1 x V1= C2 x V2 Where C1 = 100 ppm, C2 = standard ppm, and V2 = 1000 uL, V1 = the amount of 100 ppm stock that you will add. The remaining 1000 uL is added to the standards as 0.5 M K2SO4. Table. Recipe for NH4+ standards Standard (ppm) 0.5 M K2SO4 (uL) 100 ppm (uL) 0 0.1875 0.375 0.75 1.5 3 1000 998.125 996.25 992.5 985 970 0 1.875 3.75 7.5 15 30 1. 2. 3. 4. In a microcentrifuge tube add 1.5 mL of the 100 ppm stock Label microcentrifuges with the names for the 6 standards Add the calculated amount of 0.5 M K2SO4 in uL to each standard using the same pipette Add the calculated amount of 100 ppm stock in uL using a new pipette tip for each standard 5. Cap the tubes and invert them 5-times to mix the solution Analyzing soils for NH4+ Equipment and Chemical list: 96 well plate Reagent A Reagent B Pippette Pippette tips 1. Find a clean acid-washed 96 well plate. The maximum volume that one of these wells hold is 350 uL 2. Pipette 200 uL sample into each well. Make sure to fill out the data sheet to label each well 3. Load two wells with each sample and average the values also make sure there are not unexpectedly large differences between any two measures 4. Pipette in each well 50 uL of A, and 50 uL of B to each well. Make sure to save pipette tips by using one tip for A and B for an entire plate (2 tips total) 5. Cover the plate with the top 6. Record the time 7. After approximately an hour measure/record the absorbance at 650 nm. Make sure there are no bubbles in the sample. You must be trained and approved by Dr. St. Clair to use the spectrophotometer Warning: We are measuring the following ammonium concentrations: For soils with low ammonium use the following-low range, 0 to 1 ppm N, use 800 uL sample, 200 uL A, and 200 uL B. Lower limit is approximately 0.02 ppm Extra notes for soils with higher or lower NH4+ concentrations You will need to adjust these ratios so they fit in the 96-well plate For up to 10 ppm N, use 20 uL sample, 110 uL A, and 110 uL B. Lower limit is approximately 0.2 ppm N. If your soil values do not fall within this range please see below. Remember to also change your standard curve. For higher concentrations, dilute reagent A 1:1 with nanopure water and use the following sample to reagent ratios: For up to 20 ppm N, use 40 uL sample, 500 uL A, and 500 uL B. Lower limit is approximately 0.4 ppm N. For up to 60 ppm, use macrocuvets, 45 uL sample, 1500 uL A, and 1500 uL B. Lower limit is about 1 ppm N. Nitrate, NO3Preparation of NO3- Reagent4 Equipment and Chemical list: 0.5 M HCl- add 8.33 ml HCl to 200 ml DI water Vanadium (III) Chloride (remake often) Sulfanilamide N-(1-naphthyl)ethylenediamine dihydrochloride Weigh paper 200 ml container or Erlenmeyer flask 1. 2. 3. 4. 5. 6. Pour approximately 200 ml 0.5 M HCl into a bottle Weigh and add approximately 0.5 g vanadium(III) chloride Dissolve with gentle swirling (side to side shaking) Weigh and add about 0.2 g sulfanilamide Weigh and add about 0.01 g N-(1-naphthyl)ethylenediamine dihydrochloride Dissolve all chemicals by gently swirling *The reagent is stable for about a week in the refrigerator or indefinitely if frozen. If there are any problems with the standard, try to fix it by remaking the vanadium chloride. 4 These notes are from Megan Taylor, who ran this procedure for the 2012 samples. “For this procedure I made the NO3 reagent and stored half of it in the freezer and half of it in the fridge. It lasts about 1week in the fridge. Then I would unfreeze the other half and use it for about a 1 week if necessary. I put the sample liquid into microcentrifuge tubes and would use a microcentrifuge to make the white precipitate go to the bottom of the tube before extracting the sample. I created a 5 ppm standard. I would put 430ul K2SO4 into 4 of the 5 microcentrifuge tubes. Then I would mix in the 5 ppm into the tube without K2SO4. I would do this again with the next tube (meant to be the 2.5ppm standard) Then take the mixture from that tube and put it into the next tube and so on. This created standards with values of 5, 2.5, 1.25, .625, .3125, and 0 ppm. When putting the samples into the plates, I would put 150ul of the samples first. Then add 150ul of the NO3 reagent. Then I would wait 6-8 hours and use the spectrophotometer to read the samples. I created a graph from the standard ppm and abs values. I then used the regression equation to convert the absorbance values of the samples to ppm. Samples a pink color when read” For samples with NO3- concentrations higher than 5 ppm, discovered after the first run, we ran them a second time after diluting the sample 1:1 with 0.5 M K2SO4 (diluted by ½). If the diluted sample was still higher than 3 ppm we diluted it 1:2 with 0.5 M K2SO4 (diluted by 1/3). Creating a NO3- standard and standard curve Making a 100 ppm stock solution Equipment and chemical list: 500 ml volumetric Potassium Nitrate (KNO3) Parafilm 1. 2. 3. 4. Fill a 500 ml volumetric 1/2 full with nanopure water Add 0.361 g of (KNO3) Cap the top with parafilm and shake until all the chemicals are dissolved Label and store in a 500 ml glass bottle This is for a 100 ppm stock solution that is used to generate you standard curve Warning: When creating the standards and analyzing samples wear gloves Making a standard curve for soils with NO3- concentrations up to 5 ppm. This is the standard curve that we use for most wildland soils since inorganic N does not usually hang around in this form. The standard curve includes 6 standards: 5, 2.5, 1.25, .625, .3125, and 0 ppm. To calculate the standards we use the following equation: C1 x V1= C2 x V2 Where C1 = 100 ppm, C2 = standard ppm, and V2 = 1000 uL, V1 = the amount of 100 ppm stock that you will add. The remaining 1000 uL is added to the standards as nanopure. Table. Recipe for NO3- standards Standard (ppm) 0.5 M K2SO4 (uL) 100 ppm (uL) 0 0.3125 0.625 1.25 2.5 5 1000 996.875 993.75 987.5 975 950 0 3.125 6.25 12.5 25 50 1. 2. 3. 4. In a microcentrifuge tube add 1.5 mL of the 100 ppm stock Label microcentrifuges with the names for the 6 standards Add the calculated amount of 0.5 M K2SO4 in uL to each standard using the same pipette Add the calculated amount of 100 ppm stock in uL using a new pipette tip for each standard 5. Cap the tubes and invert them 5-times to mix the solutions Analyzing soils for NO3Equipment and Chemical list: 96 well plate Nitrate Reagent Pippette Pippette tips 1. Find a clean acid-washed 96 well plate. The maximum volume that one of these wells hold is 350 uL 2. Pipette 150 uL sample into each well. Make sure to fill out the Nitrate 96-well run data sheet for each name. 3. Load two wells with each sample and average the values also make sure there are not unexpectedly large differences between any two measures 4. Pipette in each well 150 uL the Nitrate reagent to each well. Make sure to save pipette tips by using one tip all the wells. 5. Cover the plate with the top 6. Record the time and wait at least 6-8 hours 7. At room temperature, color is maximum after about 6-8 hours, and stable for at least 2 days. Read absorbance at 540 nm against a reagent blank (0 ppm standard). Be sure there are no bubbles in the wells. 8. You must be trained and approved by Dr. St. Clair to use the spectrophotometer Measuring the concentration: Using the spectrophotometer -Read absorbance at 650 nm for NH4 against a reagent blank (0 ppm standard) after about an hour. -Read absorbance at 540 nm for NO3 against a reagent blank (0 ppm standard) after 6-8 or before 2 days. 1. 2. 3. 4. 5. 6. Plug in the instrument Turn on the computer Turn on the spectrophotometer machine Open Softmax pro version 5 Open Excel The tray will open automatically. Put in the plate. Push the “Drawer” button to close the tray holder. 7. Click settings and set wavelength to 650 nm (NH4) or 540 nm (NO3) 8. Push read to read samples 9. Copy the results into an Excel sheet 10. If reading more samples repeat steps 6-9. If not, remove sample. Push “Drawer” to close tray. 11. Turn off the computer 12. Turn off the machine 13. Unplug the machine 14. Cover the machine Calculating ppm N in the soil from the absorbance readings 1. Calculate the mass of soil that was extracted by finding the percent of soil in the 4 g of soil dried in the soil tin and multiplying that by the mass of the soil + water extracted a. To find the percent of soil in the tin, divide the dry mass of soil by the wet mass of soil (remember to subtract the mass of the tin to get wet and dry mass of soil) (Table 1). GramsOfSoilExtracted TinNumber WeightOfTin WetSoilPlusTin DrySoilPlusTin MassDrySoil MassWetSoil ProportionDrySoil Mass of Soil 15.04 16 1.03 5.05 4.13 3.1 4.02 0.771144 11.59801 15.07 3 1.03 5.03 4.47 3.44 4 0.86 12.9602 15.07 2 0.99 5.02 4.25 3.26 4.03 0.808933 12.19062 15 45 1.03 5.03 4.02 2.99 4 0.7475 11.2125 15.01 11 1.02 5.02 4.54 3.52 4 0.88 13.2088 Table 1. Sample calculation of soil mass 2. Use the absorbance values for the standards to create a standard calibration line (The Rsquared of this line should be 0.99 or higher) (Fig. 1). Figure 1. Example of standard line used to calculate ppm NH4 3. Use the equation of the line to calculate the concentration of NH4 and NO3 in the samples (Each set of standards and samples will produce its own standard line) 4. Multiple the ppm N in the sample by the dilution factors to calculate N in the Soil a. Calculate dilution factors using this equation: Dilution Factor = Total Volume / Aliquot Volume (measured sub-volume of the original sample) b. The first dilution factor is approximately 3 = (Mass of soil + 30 mL K2SO4) / Mass of soil c. The second dilution factor for NH4+ is 1.5 = (200 uL Sample +50 uL Reagent A +50 uL Reagent B) / 200 uL Sample d. The second dilution factor for NO3- is 2 = (150 uL Sample + 150 uL Reagent) / 150 uL Sample e. If the sample had more than 3 ppm it was diluted again, giving a third dilution factor of 2 = (100 uL Sample + 100 uL K2SO4) / 100 uL Sample f. If the diluted sample still had more than 3 ppm it was diluted again, giving a third dilution factor of 3 = (100 uL Sample + 200 uL K2SO4) / 100 uL Sample g. For the third dilution factor we can use a 1 if there was no third dilution, a 2 if the sample was diluted by half, and a 3 if the sample was diluted by one third. 5. When you multiple the ppm N in the sample by the dilution factors you should end up with ppm N in the soil, this is the value you analyze and report For Calculating Net N Mineralization rates 1. Sum the concentration of NH4+ and NO3- in the initial soil samples 2. Sum the concentration of NH4+ and NO3- in the incubated soil samples 3. Subtract the initial N values from the incubated values 4. Divide this amount by the number of days of the incubation (usually 28) 5. This gives you the net mineralization rate in ppm per day (usually reported as ug N/g soil /day) For Calculating Net Nitrification rates 1. Subtract the initial NO3 concentration from the incubated NO3 concentration 2. Divide this amount by the number of days of the incubation (usually 28) 3. This gives you the net nitrification rate in ppm per day (usually reported as ug NO3/g soil /day)