diffusion_osmosis.ver8 - RI
advertisement

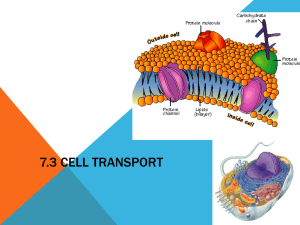
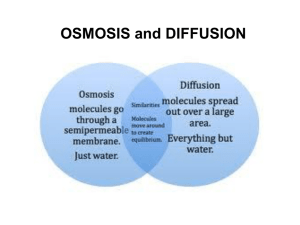
SAM Teachers Guide Diffusion, Osmosis, and Active Transport Overview Students investigate diffusion, osmosis, and active transport in order to study the movement of molecules into and out of cells. They explore the way concentration and surface area are related to the rate of ion and molecule flow across a cell membrane. They apply what they have learned to a blood cell traveling through different concentrations of oxygen. Then they explore a 3D aquapore embedded in a membrane. Finally, students explore situations where concentrations on either side of the membrane are not equal due to the use of ATP as an energy source to transport molecules against the concentration gradient. Learning Objectives Students will be able to: Compare diffusion and osmosis. Explain how concentration differences affect the overall flow of molecules. Describe the dynamic nature of equilibrium. Apply the principles of diffusion to red blood cells in a real biological system. Explain how increased surface area increases the rate of diffusion. Explore active transport by experimenting with the relationship between ion concentration and ATP concentration, and observing the formation of electrical energy. Possible Student Pre/Misconceptions Molecules move with a purpose. They “know” to move from areas of high concentration to areas of low concentration. Molecular motion stops when equilibrium is reached. Diffusion happens at the same speed and is not affected by concentration differences or surface area. Models to Highlight and Possible Discussion Questions After completion of Part 1 of the activity: Models to Highlight: Page 1 – Simple Diffusion Example o Highlight the random motion of particles and the fact that collisions change the direction of molecular motion. o Link to other SAM activities: Newton’s Laws. Review concept that atoms are in constant motion. Page 2 – Diffusion Model o Observe the overall flow of molecules from high to low concentration and point out the difference between the net flow of the molecules and the movement of individual molecules (which is random and not directed). Page 3 – Diffusion Model o Highlight what equilibrium “looks” like. It is a state of balance, but molecules are still in motion. Possible Discussion Questions: Why is it necessary for materials to be able to move across cell membranes? Can you think of some conditions that might speed up or slow down the process of diffusion? What are some of our biological processes that are possible because of diffusion of molecules? Explain the journey of a red blood cell “picking up” and “dropping off” oxygen. Start in the lungs and go from there, using your understanding of diffusion. How do you think a lack of iron in the blood would affect oxygen levels? After completion of Part 2 of the activity: Models to Highlight: Page 6 – Surface Area Model o Highlight the importance of increased surface area in the effectiveness of diffusion in biological systems. o Review the importance of surface area in both organic and inorganic systems. Page 7 – Determining Which Molecules Need Pores o Highlight the concept of selective permeability in determining which items can pass through the membrane easily. Page 8 – Osmosis o Highlight the similarities and differences between diffusion and osmosis as well as the importance of water in biological systems. Page 9 – Active Transport and the Production of Voltage o Highlight the concept of active transport and the presence of an electric potential when ions cross cell membranes. o Link to other SAM activities: Electric Current. Review electric potential and the connection to voltage. Page 10 – Chemical Energy of ATP is Converted into Electrical Energy o Point out that the chemical energy decreases every time ATP reacts with the calcium transport channel/protein, and that this pushes a calcium ion to the other side of the membrane. o By building up the concentration of positive ions to a greater degree on one side of the membrane an electric potential is built up. Possible Discussion Questions: What is the role of surface area in diffusion? What are some examples of specific biological functions that rely on increased surface area? Extensions: Correlation of how a muscle cell is like a battery; example of sodium / potassium pump. Demonstration/Laboratory Ideas: o Spray a bottle of perfume at the front of the classroom and see how long it takes for students to smell it. Discuss in terms of diffusion. o Drop food coloring into a beaker of water. Have students hypothesize what will happen. Have students draw cartoons / animations at the molecular level. Repeat with water of varying temperatures and integrate the effect of temperature on molecular motion and random collisions. o Diffusion Baggie Lab – Use starch and iodine to show diffusion. Refer to link below for possible procedure: http://www.biologycorner.com/worksheets/diffusion.htm Connections to Other SAM Activities The goal of this activity is for students to explore the concepts of passive diffusion, osmosis (and osmotic pressure), and pumping materials across a membrane against the natural equilibrium, known as active transport. From Atoms and Energy students learn about conservation of energy and in Electrostatics they learn about voltage, which is important when studying chemical and electrical potential in active transport. A background in Atomic Structure is necessary for students to understand that atoms are made of protons, neutrons, and electrons. Ions exist as a result of atoms that have gained or lost electrons. Newton’s Laws at the Atomic Scale focuses on the concept that atoms are in constant motion, moving in a straight line until they collide. This underscores the randomness of the motion involved in diffusion. Phase Change serves as a reference to the states of matter (particularly solids and liquids) that are addressed in this activity. Gas Laws highlights the random motion of gas particles, which behave much like particles dissolved in water or other solvents seen in this unit. Understanding osmotic pressure is also quite similar to the underlying principles behind gas pressure. The Solubility activity helps students understand why some things can or cannot cross membranes. Cellular Respiration highlights how ATP is used to move materials across the cell membrane. Four Levels of Protein Structure gives students background in how proteins are organized; student can thus appreciate the details about aquapores. The Molecular Recognition activity allows students to recognize that one function of proteins is to act as transmembrane molecules. Finally, in the Lipids and Carbohydrates activity, students gain background into the structure and function of lipids, which form the membranes across which the molecules are being transported. Activity Answer Guide Page 1: 1. After looking at the path taken by several red dye molecules, their motion can best be described as: (b) 2. It is often stated that substances diffuse from highly concentrated areas to areas of low concentration. Describe how this works if a molecule can't tell that it is in a region of high concentration and just diffuses randomly through collisions. The molecules are in constant motion, colliding with other molecules in the area. As they move they spread out because they are bumping into these other molecules and changing directions. Each individual molecule moves randomly, and can't tell if it’s in an area of high or low concentration. However, there are so many more molecules of that type in the highly concentrated area that there is a greater chance that one of them will move to the area of low concentration. Sample snapshot: There are many more carbon dioxide molecules outside the cell than inside the cell. 4. Set up the model so that it is IN equilibrium. Then use the "snapshot" button below the model to take a picture of your setup. Use the "open" button below to place that image here. Page 2: 1. Set up the model so that there is a high concentration of something outside and a low concentration of that same thing inside. What are the chances that a molecule will move into or out of the cell? (b) 2. Describe the flow of molecules when there is an area of high and low concentration. (c) Page 3: 1. What is true of the concentrations when equilibrium has been reached? (d) 2. What is true of the rate at which molecules move into and out of the cell at equilibrium? (c) 3. Set up the model so that it is NOT in equilibrium. Then use the "snapshot" button below the model to take a picture of your setup. Use the "open" button below to place that image here. Sample snapshot: The system is now in equilibrium. The concentration of oxygen and carbon dioxide molecules is the same inside and outside of the cell. Page 4: 1. What happens to the oxygen concentration of the cell when you move it to a new environment? (d) 2. Explain how a red blood cell delivers oxygen from your lungs to the rest of your body. The oxygen concentration is highest in the lungs, so oxygen will diffuse into the cell. In other parts of the body, the oxygen concentration is lower, so the red blood cell can "deliver" oxygen as it diffuses out of the cell to move toward equilibrium. Page 5: 1. Describe what happens when the red blood cell contains hemoglobin: (c) 2. Explain how hemoglobin helps transport more oxygen than could normally be done with simple diffusion: Iron in the hemoglobin molecule sticks to the oxygen. There are several hemes groups in a hemoglobin and each bind to oxygen.These oxygen molecules are no longer free to diffuse back out of the red blood cell. Page 6: 1. How does surface area affect diffusion rates? (d) Sample snapshot: Side view of an aquapore. 2. Take an image showing water going through the channel formed by the aquapore: 2. Single-celled organisms absorb everything they need directly through their "skin," their cell membrane. However, you could never get enough oxygen if oxygen could only diffuse through your skin. Explain why it is necessary to have lungs with large surface areas: The increased surface area of lungs allows more opportunities for oxygen to diffuse into the surrounding cells. Page 7: 1. Take an image showing a side view of an aquapore poking out of both sides of a piece of cell membrane. (Note: You will need to find the right scene on the molecule tour and rotate the molecule yourself to get the correct image.) Sample snapshot: Water traveling through the channel. 3. Which are the only types of molecules that pass easily through the cell membrane? (c) 4. What is true of most naturally occurring pores? (d) Page 8: 1. Osmotic pressure is related to salt concentrations (or other dissolved substances) in what way? (a) 2. If you want water to flow out of the cell faster than into the cell you should: (c) 3. Cells generally stay in equilibrium with their surroundings. What are two ways you know the cell has reached equilibrium? (b) (c) 4. Describe the similarities and differences between diffusion and osmosis. Diffusion and osmosis both involve random movement of molecules from areas of high to low concentration. Osmosis is specific to water molecules. Both proceed to equilibrium. Page 9: 1. What must be done to get an electric potential (a voltage) across the membrane? (c) 2. How can you get the maximum voltage across the cell membrane? (e) Page 11: 1. Which best describes the motion of an INDIVIDUAL ion or molecule? (c) 2. Describe in as many ways as possible how you know when a system has reached equilibrium: The concentration remains constant even though the molecules continue to move randomly, yet equally in all directions. The rate of molecules moving into and out of the cell will be equal. 3. Describe what will happen if a cell has an oxygen concentration that is higher outside of the cell compared with inside of the cell (see illustration to the right): (d) 4. Describe how surface area affects the speed at which diffusing ions or molecules reach equilibrium across both sides of the surface: Page 10: The greater the surface area, the greater the diffusion rate. 1. What factor most affects how much chemical energy you start with? (b) 5. Which of the following can pass through a cell membrane without a specialized pore: (c) 2. Describe how the chemical energy in ATP is converted into electric potential energy. 6. If you were to drink salt water from the ocean, which has a high concentration of dissolved ions, what would happen? (a) The ATP molecules react with the calcium transport channel to push ions across the membrane. This causes an imbalance in the charge on both sides of the membrane, creating a voltage across the membrane. The chemical energy of the ATP has been turned into electric potential energy of different ions’ concentrations. 7. Describe how you can make an electric potential occur across a membrane, and how you can maximize this potential energy: To create an electric potential you need to make the concentration of one ion different from another across a membrane. To maximize this, you should move all the positive ions to one side and all the negative ions to the other. SAM HOMEWORK QUESTIONS Diffusion, Osmosis, and Active Transport Directions: After completing the unit, answer the following questions to review. 1. During diffusion molecules move from areas of high concentration to areas of low concentration. Is this process spontaneous or directed by the cell? Explain. 2. Look at the two images below. Which one represents a system that is in equilibrium? Describe the expected motion of particles in each of the systems shown. *Note: All particles shown are carbon dioxide. 3. Give an example of a molecule that can move easily through a cell membrane. Give an example of a molecule that requires assistance to cross a cell membrane. Why are there differences in how molecules can cross the cell membrane? 4. Diffusion and osmosis refer to the movement of particles until they reach equilibrium. What is active transport? Why is this process sometimes necessary for cells? SAM HOMEWORK QUESTIONS Diffusion, Osmosis, and Active Transport – With Suggested Answers for Teachers Directions: After completing the unit, answer the following questions to review. 1. During diffusion molecules move from areas of high concentration to areas of low concentration. Is this process spontaneous or directed by the cell? Explain. Spontaneous. As the molecules move, they bump into one another and spread out from regions of high concentration to regions of low concentration. 2. Look at the two images below. Which one represents a system that is in equilibrium? Describe the expected motion of particles in each of the systems shown. *Note: All particles shown are carbon dioxide. System B is in equilibrium. The particles would still be in motion, but there would be equal amounts of carbon dioxide on either side of the membrane. In system A, there are more carbon dioxide molecules on the left of the membrane. Therefore, you would expect more molecules to move to the right until that system also reached equilibrium. 3. Give an example of a molecule that can move easily through a cell membrane. Give an example of a molecule that requires assistance to cross a cell membrane. Why are there differences in how molecules can cross the cell membrane? Only small non-polar molecules, such as carbon dioxide, can easily pass through the membrane. The non-polar molecules pass easily through the non-polar tails of the lipids following the principle of “like dissolves like.” Large molecules, ions, and polar molecules all need assistance crossing the cell membrane because the lipid bilayer is not conducive for them to dissolve. They cross with the help of a protein pore. 4. Diffusion and osmosis refer to the movement of particles until they reach equilibrium. What is active transport? Why is this process sometimes necessary for cells? Active transport refers to the process when energy, in the form of ATP, is used to move molecules across the cell membrane. The chemical energy in ATP is converted into electric potential energy to create a voltage. This is necessary to move molecules, such as ions, into and out of the cell as necessary.