Science TEKS 5.5.A
advertisement

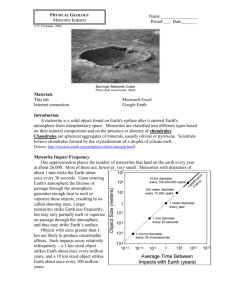
Science TEKS 5.5.A Classifying Matter Based on Physical Properties Focus Objective(s): What TEKS objective(s) does this lesson align to? Science 5.5(A) Classify matter based on physical properties, including mass, magnetism, physical state (solid, liquid, and gas), relative density (sinking and floating), solubility in water, and the ability to conduct or insulate thermal energy or electric energy. Prerequisite Objectives (if applicable): Science 3.5.(A): Measure, test, and record physical properties of matter including temperature, mass, magnetism, and the ability to sink or float. Science 3.5.(B): Describe and classify samples of matter as solids, liquids, and gases and demonstrate that solids have a definite shape and that liquids and gases take the shape of their container. Science 4.5.(A): Measure, compare, and contrast physical properties of matter including size, mass, volume, states (solid, liquid, gas), temperature, magnetism, and the ability to sink or float. Lesson Summary The students will Understand the property of density by observing a demonstration of an egg floating in water and one floating in water with salt and by observing a density column. Investigate the mass, state, magnetism, relative density, solubility and conductivity of various substances. Observe an example of the application of properties of matter by learning properties of meteorites. Assessment: What will students be able to do successfully after mastering the objective above? Guiding Questions: At least three questions that students should be able to answer after mastery of objective(s) listed above. Page 1 Science TEKS 5.5.A Classifying Matter Based on Physical Properties Understand relative density with water. 1. What is matter? Demonstrate solubility. 2. What are some physical properties of matter? Show if a substance can conduct electricity. Measure a substance’s mass. Understand that matter has different physical properties, and that these properties allow it to be identified. 3. How are meteorites identified? Engage: The first phase is to engage the student in the learning task. The student mentally focuses on an object, problem, situation, or event. The activities of this phase should make connections to past and future activities. The connections depend on the learning task and may be conceptual, procedural, or behavioral. Asking a question, defining a problem, showing a discrepant event, and acting out a problematic situation are always to engage the students and focus them on the instructional activities. Teacher Resources and Handouts for Engage section: Discrepant Event- Why does one egg float and the other egg sink? 2 500 mL beakers 3/4 full of water. Salt 2 eggs Density Column Materials You can use some or all of these liquids, depending on how many layers you want and which materials you have handy. These liquids are listed from most-dense to least-dense, so this is the order in which you pour them into the column. honey corn syrup or pancake syrup liquid dishwashing soap water (can be colored with food coloring) vegetable oil rubbing alcohol (can be colored with food coloring) lamp oil Lesson Engage section below: Discrepant Event- Why does one egg float and the other egg sink? 1. Fill 2 500 mL beakers 3/4 full of water. Page 2 Science TEKS 5.5.A Classifying Matter Based on Physical Properties 2. Add 3 tablespoons (or more if needed) of salt to one of the beakers and mix well. 3. Put an egg in each of the beakers of water. One egg should float and the other should sink. 4. Show this set up to the students without telling them what is in each beaker. Have the students draw conclusions. (Why did this occur?) Explanation The egg sank in fresh water but floated (was more buoyant) in saltwater because saltwater is denser than fresh water. Concept: Water becomes denser when salt is dissolved in it. Density Column (This activity is from http://chemistry.about.com/od/chemistryactivities/a/densitycolumn.htm ) Density Column Make a density column with many liquid layers using common household liquids. This is an easy, fun and colorful science project that illustrates the concept of density. Make the Density Column - Pour your heaviest liquid into the center of whatever container you are using to make your column. If you can avoid it, don't let the first liquid run down the side of the container because the first liquid is thick enough it will probably stick to the side of your column won't end up as pretty. Carefully pour the next liquid you are using down the side of the container. Another way to add the liquid is to pour it over the back of a spoon. Continue adding liquids until you have completed your density column. At this point, you can use the column as a decoration. Try to avoid bumping the container or mixing its contents. The hardest liquids to deal with are the water, vegetable oil, and rubbing alcohol. Make sure that there is an even layer of oil before you add the alcohol because if there is a break in that surface or if you pour the alcohol so that it dips below the oil layer into the water then the two liquids will mix. If you take your time, this problem can be avoided. How the Density Column Works You made your column by pouring the heaviest liquid into the glass first, followed by the nextheaviest liquid, etc. The heaviest liquid has the most mass per unit volume or the highest density. Some of the liquids don't mix because they repel each other (oil and water). Other liquids resist mixing because they are thick or viscous. Eventually some of the liquids of your column will mix together. During the demonstrations discuss what the students think will happen, what happened and why. Explore: Once the activities have engaged students, they need time to explore their ideas. Exploration activities are designed so that all students have common, concrete experiences upon which they continue building concepts, processes, and skills. This phase should be concrete and meaningful for the students. The aim of exploration activities is to establish experiences that teachers and students can use later to formally introduce and discuss content area specific concepts, processes, or skills Teacher Resources and Handouts for Explore section: Page 3 Science TEKS 5.5.A Classifying Matter Based on Physical Properties Lab: What's the Matter? Introduction to the Physical Properties of Matter Materials – salt, sugar, sand, penny, iron or steel nail, aluminum foil, vegetable oil, colored rubbing alcohol, balance, magnet, 2 beakers, spoon, 3 wires (stripped ½ inch on each end), battery, flash light bulb. http://ecc.hmns.org/wp-content/uploads/2013/02/Properties-of-matter.doc Background information: Purpose: The objective of this lab is to identify matter based on its physical properties. Scientific Principles: Objects that take up space and have mass are called matter. Matter is divided into the three basic states of solid, liquid, and gas. Matter is classified based on composition. Matter is identified by its characteristic properties. Physical properties are those that can be determined without altering the composition of the substance, such as color, odor, mass, density, state, conductivity, magnetism, and solubility. Lesson Explore section below: Lab: What's the Matter? Introduction to the Physical Properties of Matter Arrange students in collaborative groups. Distribute materials to each group. Explain each test before the students start working on their own. Directions – 1. Find the mass of each of the objects with the balance. Record each mass in the table. 2. Record the state of each substance in the table. 3. Test each substance for magnetism. 4. Record if each substance sinks or floats in a beaker of water. (relative density) 5. Put a small spoonful of each substance in a beaker of water, stir it gently. Record whether it dissolves in the water. 6. Use the substance, wires, battery and light to see if it conducts electricity. If it does the bulb will light. See diagram below. Page 4 Science TEKS 5.5.A Classifying Matter Based on Physical Properties Explain: Explanation means the act or process in which concepts, processes, or skills become plain, comprehensible, and clear. The process of explanation provides the students and teacher with a common use of terms relative to the learning experience. In this phase, the teacher directs student attention to specific aspects of the engagement and exploration experiences. First, the teacher asks the students to give their explanations. Second, the teacher introduces explanations in a direct and formal manner. Explanations are ways of ordering and giving a common language for the exploratory experiences. The teacher should base the initial part of this phase on the students' explanations and clearly connect the explanations to experiences in the engagement and exploration phases of the instructional model. The key to this phase is to present concepts, processes, or skills briefly, simply, clearly, and directly, and then continue on to the next phase. Teacher Resources and Handouts for Explain section: Power Point presentation- Matter: What Is It? Have the students use a Frayer model below to define each term. Page 5 Science TEKS 5.5.A Classifying Matter Based on Physical Properties Lesson Explain section below: Use the PowerPoint presentation Matter: What is It? to explain the terms – matter, mass, state of matter, magnetism, density, solubility, and conductivity. Students fill in Frayer model for each term. Elaborate Once the students have an explanation of their learning tasks, it is important to involve them in further experiences that apply, extend, or elaborate the concepts, processes, or skills. Elaboration activities provide further time and experience that contribute to learning. The teacher should provide opportunities for students to practice their learning in new contexts. Teacher Resources and Handouts for Elaborate section: Provide one copy of the information below for each group of 3-4 students. http://meteorite-identification.com/mwnews/81303.html SKAGIT VALLEY HERALD August 13, 2003 Rock that landed in Mount Vernon couple's yard may be a meteorite By KASIA PIERZGA A Mount Vernon couple might make state history if the rock they saw fall from the sky a few months ago turns out to be a meteorite. In May, Gail and Mark Fredlund were in the back yard of their home northeast of Mount Vernon watching hummingbirds zipping around a feeder on a sunny afternoon when an unusually loud, whistling buzz caught their attention. They looked up in time to watch as a rock whacked into a coil of plastic yard edging at the border of the yard, kicking up a puff of dust before it bounced back about four feet onto the grass. "It came right over our house," said Mark Fredlund. "It was going so fast, I think if it hit the roof it would have gone right through it." According to University of Washington astronomy expert Toby Smith, there's a good chance Fredlund's flying rock really is a meteorite, because the rock appears to have magnetic properties. Finding meteorites is unusual in Washington, Smith said. Most of them just look like plain old rocks, so it would be hard to spot one in the Northwest's rocky landscape. People often stop by the astronomy department with rocks they think might be meteorites. But in Smith's 10 years of teaching and doing research at UW, not one sample has turned out to be anything more than just a plain old rock. So if it turns out that the object is a meteorite, the Fredlunds may have made history as the first Washingtonians ever to see one hit the Earth. "If this really is the first time a meteor has been seen to fall, that would be unique," Smith said. Page 6 Science TEKS 5.5.A Classifying Matter Based on Physical Properties No one would be more surprised to make Washington geological history than the Fredlunds. After all, they had a hard time believing their own eyes when they saw the rock hit the ground about 3:30 p.m. on May 1. At first, they suspected mischievous neighborhood kids had thrown the rock, but they looked around and saw no one. Mark Fredlund picked up the rock — it was cool to the touch — and looked at it more closely. It didn't look like anything unusual, just a rough, irregularly shaped rock about the size of a goose egg. Some of its surface had a reddish tint, like iron ore. A black seam ran down the middle of the rock, and part of it had a brownish crust. The Fredlunds took the rock into the house and searched the Internet for Web sites offering information about astronomy. They found a site that listed several ways to tell if an object is likely to be a meteorite, including testing to see if it will attract a magnet. They found a magnet, hung it from a string, held it near the rock — and sure enough, the magnet pulled towards it. With evidence mounting, the Fredlunds called UW to ask if an expert there might be able to take a look at the rock. They hope to meet Smith and several other astronomy researchers later this month. Aside from a few friends, no one else might ever have learned about the suspected meteorite if not for a curious neighbor's phone call to the newspaper this week. According to Smith, if the rock really is a meteorite, it likely came from the asteroid belt that stretches between Mars and Jupiter, or possibly even from the moon or Mars. If it's actually a chunk from another planet, the specimen could turn out to be more than just big news — it also could mean big money. While asteroid pieces sell on Ebay for $95 per pound, rocks that fall to the Earth from another planet can sell for as much as $60,000. Until they make the trip to visit the university's astronomy experts, the mystery rock is tucked away for safekeeping — except for when friends stop by for a visit. "Everybody wants to see the space rock," Gail Fredlund said. http://geology.com/meteorites/meteorite-identification.shtml Visual Identification of Meteor-Wrongs Meteorites tend to look different from the ordinary terrestrial rocks around them. They do not contain the common earth mineral quartz, and in general do not contain vesicles. When gas escapes from cooling molten material, it creates small pinprick holes or cavities in a rock's surface. The volcanic rock pumice, often used in skin care for the removal of calluses, contains vesicles which is one of the reasons it is very light in weight. If a suspected meteorite looks like a sponge, with lots of tiny holes, it is probably volcanic rock or slag of earthly origin. Meteorite Identification: The Magnet Test Meteorites are divided into three basic groups: irons, stones, and stony-irons. Practically all meteorites contain a significant amount of extraterrestrial iron and nickel, so the first step in identifying a possible meteorite is the magnet test. Iron and stony-iron meteorites are rich in iron, and will stick to a powerful magnet so strongly that it can be difficult to separate them! Stone meteorites also, for the most part, have high iron content and a good magnet will happily adhere to them. Many earth rocks will also attract a magnet, so this is not a definitive test, but it's a good step in the right direction. Lunar and Martian meteorites, and most achondrites (stone meteorites Page 7 Science TEKS 5.5.A Classifying Matter Based on Physical Properties without chondrules) contain little or no iron and even a powerful magnet will generally have no effect on them. However, these meteorite types are so extremely rare that, as a general rule, we discount specimens that will not adhere to a magnet. Meteorite Identification: Weight and Density Iron is heavy and most meteorites feel much heavier in the hand than an ordinary earth rock should. A softball-sized iron meteorite will likely weigh five or six pounds, making it seem unnaturally dense. Imagine holding a steel ball bearing as big as a grapefruit and you'll get the idea. Visual Identification: Fusion Crust When a meteoroid (a potential meteorite) streaks through our atmosphere, tremendous heat is generated by atmospheric pressure. The surface of the rock melts and the air around it incandesces. As a result of this brief but intense heating, the surface burns and forms a thin, dark rind called fusion crust. Meteorites literally began to burn up in our atmosphere, so they tend to appear darker than the terrestrial rocks around them. Desert varnish forms on the surface of some earth rocks, particularly in arid areas, and can easily be mistaken for fusion crust by an untrained eye. True fusion crust does not occur on earth rocks. It is delicate and will weather away over time, but a freshly fallen meteorite will exhibit a rich black crust, much like a charcoal briquette. Lesson Elaborate section below: In groups of 3-4, have students read together the articles, “Rock that landed in Mount Vernon Couple's Yard May be a Meteorite,” and Meteorite Identification. Then each group is to list the physical properties used to classify meteorites. As a class discuss the physical properties that help classify them. Discuss how this information can be used to identify other substances. Evaluate At some point, it is important that students receive feedback on the adequacy of their explanations. Informal evaluation can occur from the beginning of the teaching sequence. The teacher can complete a formal evaluation after the elaboration phase. This is the phase in which teachers administer formative or summative evaluations to determine each student's level of understanding. This also is the important opportunity for students to use the skills they have acquired and evaluate their own understanding. At this point, the teacher also determines whether students have met the performance indicators. Lesson Evaluate section below Have students answer the following questions 1. What is matter? Page 8 Science TEKS 5.5.A Classifying Matter Based on Physical Properties 2. How is matter identified? 3. What are some physical properties of matter? 4. Draw a diagram to explain the 3 states of matter. 5. What does it mean if a substance is soluble in water? 6. Why did the couple in the article think that it was a meteorite that landed in their yard? 7. What physical properties do most meteorites have? 8. What kind of substances are usually good conductors of heat and electricity? 9. Why do some things sink and some things float in water? 10. Explain the density column that the teacher demonstrated in class, why did the liquids separate the way they did? Resources used in the creation of this lesson: Based on the 5E Instructional Model presented by Dr. Jim Barufaldi at the Eisenhower Science Collaborative Conference in Austin, Texas, July 2002.Adapted from description by Cornel University, 2005. Template also adapted from CSCOPE curriculum. http://chemistry.about.com/od/chemistryactivities/a/densitycolumn.htm http://geology.com/meteorites/meteorite-identification.shtml http://meteorite-identification.com/mwnews/81303.html http://www.chem4kids.com/files/matter_intro.html Page 9