Valence Bond Theory (handout)
advertisement

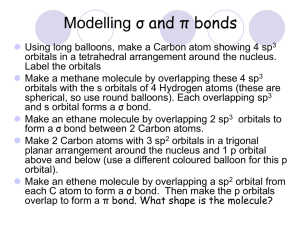
Valence Bond Theory The valence bond theory was created by Linus Pauling. According to this theory, a covalent bond is formed when two orbitals overlap (share the same space) to produce a new combined orbital containing two electrons of opposite spin. This arrangement results in a decrease in the energy of atoms forming the bonds. Figure 1: The formation of a single covalent bond in a hydrogen molecule b the overlap of two 1s orbitals of individual atoms. The two shared electrons spend most of their time between the two hydrogen nuclei. This represents a new, lower-energy state of the two atoms. Notice that the new combined orbital contains a pair of electrons of opposite spin, just like a filled orbital. Any two half-filled orbitals can overlap in the same way. The total number of electrons in the bonding orbital must be two. Figure 2: Consider hydrogen fluoride, a hydrogen atom has only one occupied orbital, the 1s orbital. The hydrogen 1s orbital is believed to overlap with the half-filled 2p orbital of the fluorine atom to form a covalent bond. This approach can also be used for larger molecules. An oxygen atom has two half-filled p orbitals. It is reasonable to propose that the 1s orbitals of the two hydrogen atoms overlap with the two half-filled 2p orbitals of the oxygen atom to produce a stable, lower-energy state. Figure 3: The two covalent bonds are created by two sets of combined s-p orbitals. The measured angle be the H-O-H bond is about 105° - this is explained by additional manipulation of atomic orbitals and a consideration of the repulsions between pairs of electrons. Overall, when atoms bond, they arrange themselves in space to achieve the maximum overlap of their half-filled orbitals. Maximum overlap produces a bonding orbital of lowest energy. Problems with Lewis Bonding Theory Problem 1 Could not explain the four equal bonds represented by the four pairs of electrons in a carbon compound like methane, CH4(g) Problem 2 Could not explain the existence of double and triple bonds. Solution to Problem 1 – Explaining equal bonds in methane Pauling and others created the idea of electron promotion from an s to an empty p orbital. Experimental evidence indicated that the electron orbitals were equivalent in shape and energy. The four bonds for carbon in molecules such as methane are explained by hybridization to four identical sp3 atomic orbitals. The two 2s electrons and the two 2p electrons form four sp3 hybrid orbitals with one bonding electron in each. This explains the bonding capacity of four for carbon. These orbitals are hybridized only when bonding occurs to form a molecule; they do not exist in an isolated atom. Figure 4: (a) An s electron is promoted to an empty p orbital in a carbon atom. (b) The four orbitals are combined to produce four hybrid sp3 orbitals. (c) Each sp3 orbital is equivalent in energy and shape. Electron repulsion requires that the orbitals are as far apart as possible – pointing to the corners of a regular tetrahedron. Pauling suggested that there were a whole series of hybridizations that could occur (Table 1). Solution to Problem 2 – Explaining Double and Triple Covalent Bonds Experimental evidence determined that three substances can be formed when two carbons atoms bonded with hydrogen – C2H6(g), C2H4(g), and C2H2(g). Lewis suggested that between the carbon atoms there must a sharing of one, two, and three electron pairs in order to obtain a stable octet around the carbon atoms. How is it possible that electrons in what we would predict as being sp3 hybrid orbitals could overlap not once, but twice or three time with just one other atom? According to the valence bond theory, two kinds of orbital overlap are possible: 1. The end-to-end overlap of s orbitals, p orbitals, hybrid orbitals, or some pair of these orbitals. This type of overlap produces a sigma (σ) bond. Think of sigma bonds as the usual single covalent bonds that you are used to drawing in structural diagrams. Figure 5: Sigma bonds form with the overlap of (a) s orbitals (b) p orbitals and (c) hybrid orbitals. 2. Two orbitals can overlap side by side to form a pi (π) bond. Pi bonds are the second and third lines in the structural diagrams for double and triple covalent bonds. Figure 6: P orbitals form with the sideby-side overlap of orbitals. Double Bonds We have already seen that the orbitals of a carbon atom can be hybridized to form four sp3 hybrid orbitals. To explain double bonds – The key new idea is a partial hybridization of the available orbitals leaving one or two p orbitals with single unpaired electrons. Double Bond Example – Ethene (C2H4(g)) Suppose that after promoting an electron in carbon’s 2s orbital to a 2p orbital, we form three sp 2 hybrid orbitals leaving one p orbital with a single electron. Still have four orbitals to form bonds but three of these are hybrids and one is a “normal” p orbital. Figure 7: Instead of mixing all four orbitals, valence bond theory suggests that only three are mixed to form sp2 hybrid orbitals and an unhybridized p orbital for a carbon atom. Figure 8: For this carbon atom, the sp2 hybrids are planar at 120° to each other and the p orbital is at right angles to the plane of the hybrid orbitals. In a molecule of ethene, the three hybrid orbitals are used to form sigma bonds between the carbon atoms and to the hydrogen atoms. The half-filled p orbitals on each carbon are believed to overlap sideways to form a pi bond. Notice that the pi bond is a region of electron density appearing above and below the sigma bond directly joining the two carbon atoms. A pi bond is a combined orbital containing a pair or electrons of opposite spin. The additional shared pair of electrons in the pi bond provides greater attraction to the two carbon nuclei, which explains why the double covalent bond is shorter and stronger than a single bond. Figure 9: (a) The sigma bond for a ethene molecule use the sp2 hybrid orbitals. (b) The two half-filled p orbitals of the adjacent carbon atoms overlap sideways. (c) The complete bonding orbitals for a ethene molecule. Triple Bond Example – Ethyne or acetylene (C2H4(g)) Focusing on the carbon atom Figure 10 describes the ground, promoted, and hybridized states of the carbon atoms that form ethyne. Figure 10: Instead of mixing all four orbitals, valence bond theory suggests that only two are mixed to form sp hybrid orbitals and two unhybridized p orbitals for a carbon atom. In ethyne, two carbon atoms bond by overlapping one of their sp hybrid orbitals, and the s orbital of the two hydrogen atoms overlap with the other two available sp hybrid orbitals. According to the valence bond theory, the unpaired electrons in the two p orbitals of the two adjacent carbon atoms share electrons by forming two pi bonds. Note that the two identical sp hybrid orbitals oriented at 180° contribute to determine the 3D orientation about each of the central carbon atoms. The result is a linear molecule for ethyne. Figure 11: (a) The sigma bonds for a ethyne molecule use the sp hybrid orbitals. (b) The two pairs of half-filled p orbitals of the adjacent carbon atoms overlap sideways. (c) The complete bonding orbitals for a C2H2 molecule.