Toxically evaluation for some sodium lauryl sulphate creams
advertisement

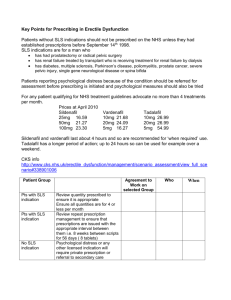
446 FARMACIA, 2008, Vol.LVI, 4 SKIN TOXICITY EVALUATION FOR SOME SODIUM LAURYL SULPHATE CREAMS. A DOSE-DEPENDENT RELATIONSHIP FOR ANIONIC SURFACTANT SIMONA ARDELEAN1, CRISTINA A. DEHELEAN2*, CODRUŢA ŞOICA2, BRÂNDUŞA DUMITRIU3, LAVINIA URŞICA1, VICTOR NĂSTASE4 1 Libro pharma S.R.L. Pharmacy, Arad University of Medicine and Pharmacy Victor Babeş Timişoara, Faculty of Pharmacy 3 S.C. Biotehnos S.A. Bucharest 4 University of Medicine and Pharmacy Gr.T.Popa Iaşi, Faculty of Pharmacy * corresponding author: criscros1973@yahoo.com 2 Abstract Surfactants represent an auxiliary substance category, as they are used in both pharmaceutical and cosmetic products; there are demands to analyse the surfactants for quality and safety. In the category of tensioactive compounds, the role of anionactive group was reevaluated and the possibilities that confer the safety of the products containing these ingredients after a long-term utilization were outlined. It is proven that the semisolid forms containing these emulsifying compounds are of high quality. The frequent use of tensioactive products leads to the noxiousness study of these compounds and the establishing of a very exact dose-effect relationship. The study tries to analyse these aspects of the noxious reactions of surfactants by modern tests: transepidermal water loss (TEWL), moistening degree, etc. The main conclusion is that tensioactives like sodium lauryl sulphate can determine important skin toxicity on concentrations higher than 10%. Rezumat Agenţii tensioactivi sau surfactanţii reprezintă o categorie de substanţe auxiliare analizată în prezent din punct de vedere al cerinţelor de calitate şi siguranţă. Ei fac parte din forme farmaceutice şi cosmetice. Dintre compuşii tensioactivi, rolul clasei celor anionactivi a fost reevaluat şi s-au conturat posibilităţile de conferire a siguranţei utilizării pe termen lung a unor produse finite cu aceşti ingredienţi, fiind demonstrat că formele semisolide realizate cu astfel de compuşi cu rol emulgator sunt de calitate superioară. Folosirea frecventă a unor forme finite cu surfactanţi impune analiza nocivităţii cutanate a acestora şi stabilirea unor relaţii doză-efect biologic foarte exacte. Studiul îşi propune să analizeze aceste aspecte ale nocivităţii surfactanţilor prin teste moderne: TEWL (transepidermal water loss), hidratare etc. Concluzia principală este aceea că tensioactivii de tipul laurilsulfatului de sodiu pot determina o toxicitate importantă asupra pielii la concentraţii peste 10%. surfactant cosmetics skin toxicity transepidermal water loss sodium lauryl sulphate FARMACIA, 2008, Vol.LVI, 4 447 INTRODUCTION Surfactants represent an auxiliary substance category. Being used in both pharmaceutical and cosmetic products, there are demands to analyse the surfactants for quality and safety [1,3]. Although surfactants are auxiliary components, they have an important role in obtaining several pharmaceutical forms (emulsions, emulsion ointment bases, etc). In order to assure the quality conditions of these forms, the absorption and activity of some associated active principles were changed [1,2,3]. In the category of tensioactive compounds, the role of anionactive group was re-evaluated and the possibilities that confer the safety of the products containing these ingredients after a long-term utilization were outlined because it is proven that the semisolid forms with these emulsifying compounds are of high quality [1,4]. From that group one of the most used compounds is sodium lauryl sulphate (SLS) or sodium dodecyl sulphate (SDS) [1,3]. The counterirritant capability of polymers or proteins on surfactants is well known [1]. The forms with mixtures of surfactants were also highly developed for an anti-irritant effect [1,7]. Anyway the toxicity of compounds with powerful tensioactive properties needs to be well observed. The actual analyse of tensioactive agents is justified by their extended use in cosmetic products [1,3,8]. The frequent use of tensioactive products leads to the noxiousness studies of these compounds [1,3]. MATERIALS AND METHODS For the patches, there were used: occlusive bandages, adhesive material for fixing the bandage, syringes and paper. The studies were developed under the ethical principles of the Declaration of Helsinki concerning testing on human subjects, including confidentiality on all records and papers. The rights, safety and comfort of tested human subjects are above all other scientific or social interests [1,4,8,9]. Before getting involved in such a study, each and every human subject has freely consented, knowing in detail every aspect of the testing. Including criteria: human volunteers, who have been informed of the particularities of the tests and have freely consented to it, clinically healthy, Caucasians. Excluding criteria: dermatological diseases which can interfere with the final evaluation, pregnancy, participation in other simultaneous studies or in a short period of time, tattoos, sunburns, scars in the tested areas. 448 FARMACIA, 2008, Vol.LVI, 4 Total number of volunteers were 25, 5 volunteers for each group participated to one category of tested formula. Also, participants will be excluded from the tested group if: - they do not follow investigator’s instructions; - they get ill or traumatized during tests; - they no longer agree to the study. Test description. Testing methodology consists of the following steps: a certain amount of product (2 mL) is applied with a 2 mL syringe on an occlusive bandage; the occlusive bandage impregnated with the cosmetic product is applied on the human skin (shoulder or the upper back side); simultaneously, placebo (no active substances) and positive control (sodium laurylsulphate) patches are applied on the skin; the three types of samples are coded and randomly applied by the investigator, in a “single blind” trial; the occlusive bandage is maintained with no humidity for 24 hours, keeping under observation any eventual side effect; after 24 hours the occlusive bandage is removed from the skin and the product is washed away with no rubbing; all the skin side effects are evaluated (erythema, dryness, edema) by COLIPA scores, mentioned below; any reaction is observed immediately and after 30 min, one hour, 24 and 48 hours from the removal of occlusive bandage; the evaluation is performed by specialized personnel and under the same light spot. Evaluation of toxic/irritant potential for tested cosmetic products are being performed visually – redness and/or dryness of the skin is evaluated on a scale according to COLIPA scores (Table I). Table I COLIPA scores Erythema Dryness (appearance of Edema scuams) 0 = no erythema 0 = no side effect = absence of edema 0.5 = minimal erythema 0.5 = dryness of the skin + = presence of edema without scuames 1 = diffused, spoted, 1 = minor scuams light redness 2 = reduced, uniform 2 = reduced descuamation redness 3 = strong, uniform 3 = severe descuamation, redness with big scuams 4 = extreme redness FARMACIA, 2008, Vol.LVI, 4 449 Determination of transepidermal water loss as indicator of skin toxicity was used as an instrumental method which detects the quantity of transepidermal evaporated water on surface unit, by evaluation of vapors gradient (co-ci) in an open room, based on Fick’s diffusion law (J= kD(coci)/h). Transepidermal transportation consists of the crossing of the intact corneous layer (SC). There are two crossing possibilities: intracellular and intercellular (through and among corneocytes). The crossing way depends on the partition coefficient k of the active substance: hydrophilic substances prefer the intracellular way while the lypophillic substances choose the intercellular way; in the same time, there are molecules that can go both ways. This method represents an indirect evaluation of the integrity of the hydrophilic layer responsible of the barrier function of the skin. There is a continuous diffusion of water from the human body towards the corneous layer and from there to the surrounding medium. Small levels of transepidermal evaporated water show a better function of the skin as barrier and a smaller loss of natural moistening degree. The barrier function is easily disturbed by mechanical or chemical problems. TEWL (transepidermal water loss) measurement will be performed only after using the products under occlusive bandages for 24 hours. Test type was noninvasive method of skin analysis, in a “single blind” trial. The prepared semisolid formula, for evolution TEWL tests were compared with a similar one reached with a dry birch tree outer bark extract, 1g/100 g cream (Table II). That formula proved a protective activity for skin [6,10]. Table II Semisolid formula used for sodium lauryl sulphate test INGREDIENT TYPE CONCENTRATION (mg/100 g base) Sodium lauryl sulphate (SLS) xg Cethylic alcohol 8g Cocoa butter 7g Vaseline 25 g Preservative solution 60 g RESULTS AND DISCUSSION First observations about the irritancy potential of semisolid formulations were visual evaluations. The main tests were: erythema, desquamation and edema. The results are presented in Table III. 450 FARMACIA, 2008, Vol.LVI, 4 Table III The evaluation of irritancy potential (24 h patch tests after COLIPA regulations). A visual evaluation Tested sample Cream base with vegetal extract Erythema Desquamation Edema % subjects with the effects 0 0 75% SLS 1% 0.5 0 - 80% SLS 10% 1 0 - 75% SLS 20%` 3 0 + 80% Nontreated witness 0 0 - 100% Observations The rest (25%) of the subjects presented slight redness, edema, which disappeared in one hour after the removal of the patch 20% from the tested subjects presented or edema, or diffuse redness, symptoms that disappeared after 1 hour after removing the occlusive patches 25% of tested subjects had edema or diffuse redness, symptoms that disappeared 1 hour after removing the occlusive patches The product obviously irritates the applied skin surface, erythema persisted for 24h, and the edema disappeared only after minimum 2 hours from the patches removal - From the presented data it can be observed that the concentration of SLS is very important for the noxious effect on the skin. Concentrations over 10% are toxic for the skin even in an acute evaluation. The most evident adverse effects are: persistent erythema, evident edema and no evident desquamation. 451 FARMACIA, 2008, Vol.LVI, 4 30 25 20 15 TEWL (g/h m 2) 10 5 0 Base SLS 1% SLS 10% SLS 20% Nontreat Figure 1 TEWL values evolution after SLS creams application comparing to a cream base. A dose-dependent relationship TEWL general values (figure 1) confirm possible damages and adverse effects for a semisolid formula with a concentration over 10% of SLS. TEWL values for a 20% SLS cream are around 30% that represents important changes in moistening degree of the skin. The evolutions of TEWL parameters for not very harmful formula are presented in Table IV. Table IV TEWL evolutions for the SLS creams. A time-dependent relationship for not very noxious concentrations of SLS Tested sample % TEWL variation after 30min +20% % TEWL variation after 1 h % TEWL variation after 3 h % TEWL variation after 5 h +20% +17% +7.4% 3. SLS 1% -3.75 +10% +3% No variation 4. SLS 10% -35% -70% -15% No variation 1. cosmetic cream with 1% SLS and birch tree extract Observations Integrity of hydrolipidic layer was improved immediately after cream application (TEWL decrease with 20%), effect that doesn’t persist more than 3 hours. This concentration of SLS doesn’t change significantly the skin barrier function. It is noticed the hydrolipidic layer degradation correlating with an increasing of the transepidermal evaporated water in the first hour after product application and the activity of some homeostatic mechanisms. 452 FARMACIA, 2008, Vol.LVI, 4 From the transepidermal water loss (TEWL) variation we may notice the increasing of variation and a status position after a few hours for tested creams. Negative values indicated decreasing of moistening degree and positive values improving of moistening degree by transfer of water from the cream base. Any formula improved with vegetal extracts that offer skin protection/hydration leads to increasing of moistening degree. CONCLUSIONS Sodium lauryl sulphate (SLS) is a well known surfactant that is used as a model of irritation. It determines important toxic effects on skin, dependent on a dose-effect relationship. Its primary noxious effects consist of erythema and edema. Concentrations over 10% are very harmful for the skin, in particular when it is applied as single compound. Any improving of the formula with protective compounds reduces the surfactant noxious effect and first of all, TEWL negative variation. REFERENCES 1. Barel A.O., Paye M., maibach H.I., Handbook of Cosmetic Science and Technology, 2003, 271-275 2. De Paepe K, Lagarde J-M., Gall Y., Roseeuw D., Microrelief of the skin using a light transmission method, Arch. Dermatol. Res., 2000, 292, 500-510 3. Dragomirescu A., Dehelean C., Dermatofarmacie si cosmetologie, Ed. Brumar, Timişoara, 2002, 151-157, 160-172 4. Frosch P.J., Kurte A., Efficacy of skin barrier creams, Contact Dermatitis, 1993, 28, 154-162 5. Pinnagoda J., Tupker R.A., Agner R., Guidelines for transepidermal water loss measurement, Contact Dermatitis, 1990, 22, 164-178 6. Patocka J., Biologically active pentacyclic triterpenes and their current medicine signification, Journal of Applied Biomedicine, 2003, 1, 7 – 12 7. Seiller M., Martini M.C., Formes pharmaceutiques pour application locale, Technique et Documentation, Paris, 1996 8. York M., Griffiths H.A., Whittle E., Basketter D.A., Evaluation of human patch test for the identification and classification of skin irritation potential, Contact Dermatitis, 1996, 34, 202-212 FARMACIA, 2008, Vol.LVI, 4 453 9. Wissing S.A., Muller R.H., The influence of solid lipid nanoparticles on skin hydration and viscoelasticity –in vivo study– Eur.J.Pharm.Biopharm., 2003, 56(1), 67-72 10. L.Urşica, C.Dehelean, C. Peev, V.Vlaia, G.Coneac- Formulation and quality evaluation of some topical semisolid preparations containing dried extract of birch tree, Recent Developments in Pharmaceutical Analysis, Sept. 2005, 207.