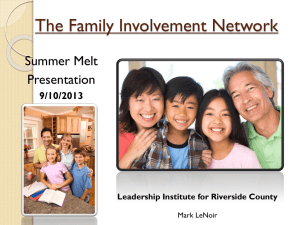
Fig. 6. Typical circuits with high magnetic permeability
advertisement

THE INVESTIGATION OF KINEMATIC VISCOSITY OF LIQUID COBALT-BASED ALLOYS B.A.Baum, G.V.Tyagunov, V.S.Tsepelev, V.Y.Belozerov, E.E.Baryshev, V.V.Konashkov Institute of Liquid Metals Physics, Urals State Technical University, 19 Mira str., Ekaterinburg, 620002, Russia ABSTRACT. The Co-B-Si system is a base of modern amorphous alloys with uncial magnetic properties. It has been studied the 12 specimens with different compositions. The method of oscillations going out of a crucible with the melt has been used for investigation of kinematic viscosity. The measurements have been made during isothermic holdings at different temperatures from 1200 to 1700oC at liquid state. It has been founded the instability of values of kinematic viscosity from holding time in this temperature interval. It has been founded that heating above some stabilization temperature (critical temperature) leads to disappears of viscosity instability. The degree of viscosity instability and stabilization temperature are defined by the heating rate of the specimen before melting and initial charge material size. This facts inform about significant changing of liquid structure. The results of the investigations are recommended to improvements to amorphous alloys melting technology. Competiteveness of that method is in the stability of amorphous tape properties from melt to melt. Cheaper charge materials substituting expansive Co-based were suggested by the authors. The material of magnetic circuit has unique technical properties: initial permeability is more than 100 000, maximal permeability is less than 600 000, magnetostriction is around null. The magnetic circuit have excellent plastic properties. The complete working life of magnetic circuit is 30 years and guaranteed storage time is 15 years. INTRODUCTION Melts, as any other thermodynamic systems, should be divided in two groups: - nonequilibrium, i.e. preserving some features of structure of initial solid phase for some time; - equilibrium, which structure and properties are determined not by pre-history but by composition and external parameters. From the point of view of their origin both groups dependent the initial crystal state. The first group is combined by means of nonequilibrium elements of its state. The second one is combined by the interatomic interaction and by the local order. The interest to these systems is stimulated by following experimental facts: - properties of molten samples of the same composition depend on pre-history (initial materials, heating rate and temperature); - these melt properties are time - instable; - non-coincidence of properties while heating and subsequent cooling. Both types of melts are connected with the initial crystalline state. The former – by the nonequilibrium features of their structure, and the latter – by the nature of interatomic associations and the nearest order formed (1-3). 1 - 85 2. NONEQUILIBRIUM MELTS Free energy of the nonequilibrium system F = U – TS is higher than its equilibrium value Fmin because the entropy of well-ordered areas inherited from initial materials appears to be lower than the equilibrium Smax. The stimuli to study these states evident from the thermodynamical point of view include the well-known experimental facts (1, 2): Property values of liquid specimens with equal chemical composition are dependent on their prehistory (initial materials, heating speed and temperatures); Property values of these melts are instable with time; Property values obtained during heating and consequent cooling of the specimens do not coincide. If a relaxation process of nonequilibrium states occurs, it often lasts for hours. Metastable states are registered more often than usual, from which the system cannot get out spontaneously, without thermal or mechanical influence. In this case, additional heating up to the fixed temperature is the simplest way to make the melt balanced. This threshold character of the system transition into the equilibrium state is indicative of the kinetic rather than diffuse regime of this process, which was confirmed by special investigations. The conditions of the experiment included: the speed of temperature changes, heating inertia of the device, impurity content variations etc - all this significantly affects the disordering process kinetics and thus the location of experimental points on the property - temperature dependence. Every multi-component system is characterized by its own nonequilibrium formations the nature of which is still to be discovered in every particular case. So far it is clear that of all nonequilibrium states the solidified ones result in instability of the structure and properties of solid specimens under formation. Thus, thanks to fundamental investigations of metal melts properties and structure, a new applied trend has appeared, i.e. development of guidelines for transforming multi-component metal melts into the balanced state with the aim of increasing and stabilizing metal product quality. 2. EQUILIBRIUM MELTS As an example let's consider widely spread iron, nickel, chrome and other 3dtransitional metals. Their "transition", implying the presence of the d-level closely located to the S-level of the upper layer, provides the opportunity for the dual behavior of d-electrons tending to participate in metal associations during condensed body formation, and at the same time as highly localized near its atom, i.e. create associations close to covalent by their properties. The electron structure in metal alloys doesn't undergo significant changes under higher temperatures and transition via liquidus line. This is shown by multiple experimental data on magnetic susceptibility, electrical conductivity and other properties associated with peculiarities of the system short-range order. Therefore the liquid state retains the transition metal atom ability to form connections of various chemical types and the opportunity to change these types depending on particular conditions melt existence. After melting the systems with coexisting connections of various types retain the capacity to change the short-range order structure. These changes, though they are 1 - 86 taking place within the same phase, have certain features simulating some polymorphic transformation attributes. In the meantime their structural changes are significantly less marked than those in the solid state and occur mainly at highest and lowest temperatures, similar to unclear phase transitions. However, the structuralsensitive properties of the melt can undergo significant anomalies here. Without a detailed description of the balanced melts properties, we can add that the character of the concentration and time dependence of their properties often coincides with similar dependence for crystalline specimens. This gives evidence to the identical mechanism of impurities influence on the solid and liquid phase structure and properties. Thus, the condensed state unity implies a significant role of one and the same interparticular attraction forces within the whole temperature range of this state existence. Maintaining the influence of interparticular forces and their specific features (e.g. chemical connection type) on mutual location of neighbouring atoms in space remains an important factor of the melt structure and property formation in relatively low overheating it above melting temperature (Tm). 3. QUASICHEMICAL MODEL STRUCTURE OF LIQUIDS OF THE MICROINHOMOGENEOUS It is expedient to discuss these problems within the framework of the above model, presenting a result of Stewart's, Frenkel's and Eiring's (1, 2) views. This version is true indeed for the transition metals, becaus any of the purestmelt of such an element owing to its ambivalent nature could be consied as a particular version of the model. It's main concept is as follows: a) The melt is composed of microregions, i.e. clusters, where the atomic arrangement is determined by a certain order (short-range). b) Owing to relatively strong thermal motion of the particles, the clusters do not have any clear boundaries. For the same reason the lifetime of the clusters is limited and depends on the energy of the chemical bonds in them as well as on the temperature. The different clusters of two and more types of ordering can exist. c) Energy inequality of interatomic interaction of different types is the cause for generating clusters of different composition and structures having dissimilar stabilities with time. d) This model takes into account energy field peculiarities of the melt atoms. All these concepts and ideas described about the melt structures having impurities are steel in progress. Our experiments constantly result in new data which can hardly be explained. For example, we might refer here to oscillating and even more complicated types of concentration, temperature and time dependences of melt properties. All this needs profound studies while developing the model. 4. EXPERIMENTAL RESULTS The Co-B-Si system is a base of modern amorphous alloys with uncial magnetic properties. It has been studied the 12 specimens with different compositions (Fig 1). The method of oscillations going out of a crucible with the melt has been used for investigation of kinematic viscosity. The measurements have been made during isothermic holdings at different temperatures from 1200 to 1700oC at liquid state. It has been founded the instability of values of kinematic viscosity from holding time in this temperature interval (Fig. 2). It has been founded that heating above some 1 - 87 stabilization temperature (critical temperature) leads to disappears of viscosity instability. The degree of viscosity instability and stabilization temperature are defined by the heating rate of the specimen before melting and initial charge material size. This facts inform about significant changing of liquid structure. Fig. 1. The kinematic viscosity of Co-B-Si alloys. a – about 1500оС, b- 1400оС 5. THE OPTIMIZATION OF PRODUCTION TECHNOLOGY The typical temperature dependences of kinematic viscosity (), electrical resistivity (), magnetic sucsecibility (), surface tension () and density (d) of the melt containing high amount of element – amorphizator is shown on Fig. 3. It is founded that the special critical temperatures named tc are determined on base of such data. 1 - 88 Fig. 2. The typical oscillations of kinematic viscosity of industry alloy during isothermic holding about1300oC The heating above tc leads to more homogenous melt state and, as a rule to forming the physical properties hysteresis (2). The overcooling value t is measured for this equilibrium melt. The presence of hysteresis is indicated the differencies between the initial and finished melt state and wishes to each the tc during melting. Some times the melt may be heated only to the anomaly temperatures tan. The melt holding time at the maximal heating temperature is experimentally during the investigation of the time dependence of properties changes at this temperature. So, the kinematic viscosity (Fig. 4) of nanocristalline magnetic Co-based alloy, has been studied (4). The first regime is heating to the temperature below tc, and further amorphisation. The second regime is heating to the temperature above tc, then amorphisation by the first regime. The third regime is heating to the temperature above tc, then overcooling and further amorphisation. The physical characteristics of amorphous ribbons were tabulated. The best service properties of ribbons were obtained by the third regime. The analysis of the results obtained while improving production technology of the amorphous Co-based alloys at the Gammamet plant using the Sirius device with the top casting weight of 50 kg shows that the parameters of all production stages of the amorphous ribbon are interconnected. An alteration of any parameter at the initial stage results in changing the whole interrelation chain and the yield will have a significant deviation from the prescribed characteristics of the strip. Besides, the parameter effect could be multiple. Thus, for instance, in an attempt to get a thinner ribbon (to avoid its fragility) they increase the disc revolving speed, but this decreases 1 - 89 the time of the ribbon contact with the cooling bed. This in its turn elevates the temperature of a yielding ribbon and makes it more fragile. Thus, all parameters of Fig. 3. The typical politerms of physical properties of amorphous alloys: t - a value of overcooling; tan – the temperature of anomalies; th – the temperature of hysteresis; tc – the critical temperature amorphous ribbon production should be optimized with the account of their interrelations. It should be noted that structural features of the devices should be referred to their technological parameters. These include the hardening disc diameter and cooling mechanism, the structure of pouring nozzle with melting crucible and some others. The major difficulty in obtaining amorphous magnetic Co-based alloys is their increased viscosity in presolidus area. This is the reason of severe turbulence of the melt flow through the nozzle. During amorphous ribbon production using the Sirius device the following problem arise (Fig. 5). The Sirius structural peculiarity is this: liquid metal goes a long way in the slide valve opening and in the nozzle after it leaves the crucible and before it comes to the quenching drum. On his way the melt doesn’t get into the inductor activity area due to which its temperature decreases uncontrollably. High viscosity melts may fail to be poured. This problem also exists 1 - 90 in soft magnetic alloys of Co-base type. The alloy has increased viscosity, twice that of the steel after melting, which is 7-1010-7 m2/sec. Fig. 4. The politerm of kinematic viscosity of the amorphous Co-based alloy: heating; o – cooling; tc – critical temperature. The numbers about the curves are the termal diagrams of three regimes Furthermore, the temperature dependence (t) has one more peculiarity, i.e. abnormal viscosity at critical temperature. When heated to t < tc, heating and cooling branches coincide. Heating up to t tc results in hysteresis which means that the melt in this case transforms into a equilibrium and more homogeneous state. This happens because immediately after melting the liquid melt presents nonequilibrium and nonhomogeneous system. It maintains some areas with atom coordination inherent to (typical for) crystalline specimen, and which are in dynamic balance with the surrounding more homogeneous liquid. Their total dissociation and the melt transition into the homogeneous state occurs at heating above critical temperature. In this case 1 - 91 atom coordination changes significantly. This conclusion is proved by experimental findings regarding surface tension (), density (d) and electrical resistance (). Fig. 5. Interrelation between production parameters and their effect on amorphous ribbon characteristics The Institute of Metal Liquids Physics has accumulated a vast experience of studying physical properties of multi-component alloys based on iron, nickel and cobalt in liquid and solid states (4). The Institute has a unique equipment including devices for alloys precision melting, the study of physical properties of materials in liquid and solid states, the thermal and differential-thermal analysis of metal transformation during heating and cooling, and equipment for general and fractional gas content. Scientific principles of producing nanocrystralline matrerials of Co-base type with unique technical magnetic characteristics have been developed. The best toroidal magnetic circuits have initial permeability of 100 000, coercivity force no more than 1,0 A/m, maximum permeability of 600 000 magnetic induction of at least 1,2 T at 800 A/m. Typical circuits produced by Gammamet is shown on Fig. 6. 1 - 92 Fig. 6. Typical circuits with high magnetic permeability CONCLUSION 1. Both scientific and applied approaches to the problem of liquid substance structure should be based upon experimental findings referring to a particular liquid and should take into account its temperature range, previous history, as well as the main purposes of the study. 2. In the temperature range close to crystal-liquid phase transition, when studying the liquid and solid phase interrelation of one and the same substance, particularly of a complex nature, the base for studying (and modeling) the melt structure could be the concept of the condensed state unity. It includes the statement of maintaining specific forces of particle interrelation and their significant role, alongside with thermal movement, in the structural system formation in the whole sphere of condensed state. 3. When studying the interrelation of properties and structure of real Co-based melts distinguished by complex ratio between valence electron localization and collectivization, with those of corresponding solid materials, the most adequate is the 1 - 93 quasichemical model microheterogeneos melt structure. The usefulness of the latter is in the opportunity to prognose the results of physico-mechanical effects on the melt and final solid product properties. 4. Liquid metal is not equilibrium after solid-liquid phase transformation. Every melt changes its structure very intensively under certain temperatures. Heating up to these temperatures contributes to the system transition into the equilibrium state or the one close to it. 5. Critical temperature values are determined in laboratories by studying temperature dependence of melt properties. These values do not depend on the value of the volume studied, because they are determined by the process at the micro level in the kinetic regime. ACKNOWLEDGEMENTS We thank the Russian Foundation for Basic Research (grant 01-02-96434) and U.S. Civilian Research and Development Foundation (grant REC-005) for support of the research. REFERENCES 1. Baum B.A. Metallic Liquids. Moscow, Science, 1979. 2. Baum B.A., Khasin G.A., Tyagunov G.V. and other Liquid steel. Moskow, Metallurgy, 1984. 3. Vatolin N.A., Pastukhov E.A. Diffraction investigation of high temperature melt structure. Moskow, Science, 1980. 4. The special technology of amorphous materials production / V.S.Tsepelev, B.A.Baum, G.V.Tjagunov and other - Proceedings International Conference on Mathematical Modeling and Simulation of Metal Technologies, MMT-2000, Ariel, Israel.-2000. 1 - 94