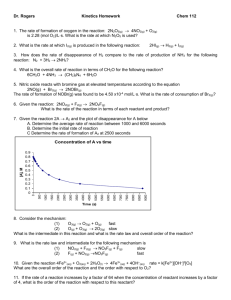
If A?B is a first order reaction and the concentration of A falls from
advertisement

If A?B is a first order reaction and the concentration of A falls from 0.050 M to 0.015 M after a period of 20 minutes, what is the rate constant, k, for this reaction? A) -0.060 min-1 B) 17 min-1 C) 380 min-1 D) 2.6 x 10-6 min-1 E) 0.060 min-1 2. Which of the following plots will not be linear? A) [A]t versus time for a first order process B) [A]t versus time for a zero order process C) ln [A]t versus time for a first order process D) ln k vs 1/T E) All of these relationships are linear. 3. What is the rate law for the reaction A + B + C ? D, if the following data was collected? Experiment No. 1 0.1 0.2 0.3 230 2 0.2 0.4 0.3 460 3 0.3 0.6 0.6 620 4 0.4 0.2 0.3 920 A) rate = k[A][C] B) rate = k[A]2[C]2 C) rate = k[A][C] D) rate = k[A][C]3 E) rate = k[A]2[C]2 4. The reason for an increase in the rate of reaction at higher temperatures may be due to: I. an increase in the number of collisions between reactants. • II. a lower activation energy for the reactions. • III. an increase in the average kinetic energy of the reactant molecules. A) I. B) II. C) I, II and III are correct. D) both I and III are correct. 5. The rate law for the reaction: 2 NO2 (g) + F2 (g) ? 2 NO2F (g) is rate = k(NO2)(F2) Which of the following mechanisms provides the best explanation of the observed rate law? A) 2 NO2 + F2 ? 2 NO2F (one step) B) NO2 + F2 ? NO2F + F (fast) NO2 + F ? NO2F (slow) C) NO2 + F2 ? NO2F + F (slow) NO2 + F ? NO2F (fast) D) F2 ? 2 F (slow) 2 NO2 + 2 F ? 2 NO2F (fast) E) None of these mechanism explains the observed rate law. 6. Consider the following reaction: 2 NO(g) + Br2(g) 2 NOBr(g) for which the following data was obtained [NO] (M) [Br2] (M) initial rate of reaction (M/s) 1 0.025 0.040 1.2 2 0.025 0.080 2.4 3 0.025 0.120 3.6 4 0.050 0.040 4.8 5 0.075 0.040 10.8 The rate law for this reaction would be: A) zero-order in Br2 B) half-order in Br2 C) first-order in Br2 D) second-order in Br2 E) None of the above. 7. How many grams of sodium carbonate are needed to make 250 mL of 0.400 M sodium carbonate solution? A) 10.6 g B) 8.37 g C) 106 g D) 40.0 g 8. The rate law for a reaction involving only A and B is 2nd order in A and 3rd order in B, respectively. If the concentration of A is doubled and the concentration of B is tripled the new rate will be: A) 6 times faster. B) 12 times faster. C) 36 times faster. D) 108 times faster.. E) 324 times faster. 9. Suppose a reaction has the following rate law: rate = k[A]1/3 Which of the following can be concluded about the reaction? A) The reaction mechanism has multiple steps. B) Three molecules of A react with every molecule of B. C) Three molecules of B react with every molecule of A. D) Molecule A reacts at three times the rate of Molecule B. 10. Which statement is true? A) The rate of appearance of products of a chemical reaction is always equal to the rate of disappearance of reactants. B) If a reaction follows a second-order rate law, it must have two steps in its reaction mechanism. C) The half-life for a first-order reaction is always larger than the half-life for a second-order reaction. D) The half-life for a first-order reaction is independent of the initial concentration of the reactant. E) The half-life for a second-order reaction is independent of the initial concentration of the reactant. 11. What is the correct rate law for the reaction: 2 NO (g) + O2 (g) ? 2 NO2 (g)? A) rate = k[NO]2 B) rate = k[NO]2[O2] C) rate = k[NO][O2] D) rate = k[NO]2 E) The rate law is impossible to determine from the information given. 12. If the reaction rate is tripled when the temperature of the reaction is increased from 150 K to 160 K, what is the activation energy of the reaction? A) 3.50 kJ/mol B) 9.60 kJ/mol C) 14.3 kJ/mol D) 21.9 kJ/mol E) 39.1 kJ/mol 13. A certain physiologically important first order reaction has an activation energy equal to 40.0 kJ/mole at normal body temperature (37oC). Without a catalyst, the rate constant for the reaction is 5.0 x 10-4s-1. To be effective in the human body, where the reaction is catalyzed by an enzyme, the rate constant must be at least 2.0 x 10-2 s-1. If the activation energy is the only factor affected by the presence of the enzyme, by how much must the enzyme lower the activation energy of the reaction to achieve the desired rate? A) 9.5 kJ/mol B) 38 kJ/mol C) 78 kJ/mol D) 140 kJ/mol E) 730 kJ/mol 14. A first-order reaction has the rate constant of 0.30 s-1. Determine the time it takes to decrease the reactant concentration from 0.6 M to 0.3 M. A) 0.60 s B) 2.3 s C) 5.5 s D) 2.0 s E) 6.0 s 15. The rate constant for a particular reaction is 1.3 x 10-4 M-1s-1 at 200 K. What is the overall order of the reaction? A) 0 B) 1 C) 2 D) 3 E) 4 16. The mechanism for the formation of product X is: • A + B ? C + D • B + D ? X Indicate the intermediate for this reaction: A) A B) B C) C D) D E) X 17. What is the volume of 8.0 g of oxygen at STP? A) 0.011 L B) 0.36 L C) 3.6 L D) 5.6 L E) 11 L 18. Consider the following reaction: 2 NO(g) + Br2(g) 2 NOBr(g) for which the following data was obtained [NO] (M) [Br2] (M) initial rate of reaction (M/s) 1 0.025 0.040 1.2 2 0.025 0.080 2.4 3 0.025 0.120 3.6 4 0.050 0.040 4.8 5 0.075 0.040 10.8 The magnitude of the rate constant, k, for this reaction is: A) less than 10-4 B) between 10-4 and 10-2 C) between 0.01 and 100 D) between 100 and 10,000 E) more than 10,000 19. For the following reaction: 4 NH3 + 7 O2 4 NO2 + 6 H2O. The substance that is formed or removed the fastest is: A) NH3 B) O2 C) NO2 D) H2O E) All substances have the same rate for appearance/disappearance. 20. The overall rate of a chemical reaction is determined by: A) the slowest step in the reaction mechanism. B) the first step in the reaction mechanism. C) the fastest step in the reaction mechanism. D) the last step in the reaction mechanism. E) the number of chemical steps in the reaction mechanism. 21. Which of the following statements is false about a catalyst? A) Catalyst in living systems are called enzymes. B) A catalyst does not change the equilibrium constant for a reaction. C) A catalyst does not change the activation energy of a reaction. D) A catalyst does not undergo any net chemical change. E) A catalyst does not change the rate law for a reaction. 22. Given the following information: • Reaction I: H = -100 kJ/mol; Ea = 10 kJ/mol • Reaction II: H = - 50 kJ/mol; Ea = 30 kJ/mol • Reaction III: H = 100 kJ/mol; Ea = 20 kJ/mol • Reaction IV: H = 50 kJ/mol; Ea = 15 kJ/mol Which reaction will occur at the fastest rate? A) I B) II C) III D) IV 23. Consider the following reaction: 2 NO(g) + Br2(g) 2 NOBr(g) for which the following data was obtained Experiment No. [NO] (M) [Br2] (M) initial rate of reaction (M/s) 1 0.025 0.040 1.2 2 0.025 0.080 2.4 3 0.025 0.120 3.6 4 0.050 0.040 4.8 5 0.075 0.040 10.8 The rate law for this reaction would be: A) zero-order in NO B) half-order in NO C) firstorder in NO D) second-order in NO E) None of the above. 24. The decomposition of ether at 504 °C is a first order reaction with t½ = 27.0 min. What is the rate constant for this reaction? A) 0.0257 min B) 0.0257 min-1 C) 0.0177 min D) 0.0177 min-1 E) None of the above. 25. Which of the following statements is true? A) The activation energy always increases as temperature rises. B) The activation energy always decreases as temperature rises. C) The rate constant always decreases as temperature rises. D) The rate constant always increases as temperature rises. E) The rate constant always increases as the activation energy increases.