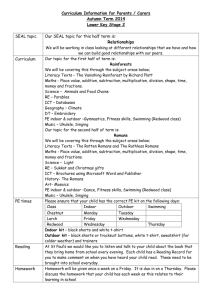
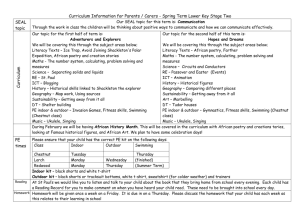
Identification and Classification
advertisement

IDENTIFICATION, CLASSIFICATION, AND CORRELATION OF ULTRAFINE INDOOR AIRBORNE PARTICULATE MATTER WITH OUTDOOR VALUES By Nick Allan Facciola B.S., Mechanical Engineering University of California, Santa Barbara, 2004 A thesis submitted to the Faculty of the Graduate School of the University of Colorado in partial fulfillment of the requirements for the degree of Master of Science Department of Mechanical Engineering 2006 This thesis entitled: Identification, Classification, and Correlation of Ultrafine Indoor Airborne Particulate Matter with Outdoor Values written by Nick Allan Facciola has been approved for the Department of Mechanical Engineering ______________________________________________________ Dr. Shelly L. Miller ______________________________________________________ Dr. John Zhai ______________________________________________________ Dr. Darin Toohey Date _________________ The final copy of this thesis has been examined by the signatories, and we find that both the content and the form meet the accepted presentation standards of scholarly work in the above-mentioned discipline. ABSTRACT Facciola, Nick Allan Master or Science, Department of Mechanical Engineering Identification, Classification, and Correlation of Ultrafine Indoor Airborne Particulate Matter with Outdoor Values Thesis directed by Professor Shelly L. Miller Many studies report that adverse health effects are most strongly correlated with fine particulate matter (< 2.5 micron in diameter), originating from ambient emissions mainly derived from fossil fuel combustion. This study examines the infiltration of ultrafine (< 0.1 micron) and fine particulate matter into indoor environments including typical office space as well as elementary schools. With the use of an Ultra High Sensitivity Aerosol Spectrometer and an Aerosol Mass Spectrometer, the size and chemical speciation of ultrafine and fine particulate matter can be found, providing data that can be used to compare and correlate indoor and outdoor particulate matter concentrations. First and foremost, this study provides important data needed to understand exposure and health risks associated with inhalation of fine particulate matter. In addition, the information provided by this study can improve understanding of filtration requirements in mechanically ventilated buildings. The correlation of indoor to outdoor particulate concentrations can be used to evaluate the performance of heating, ventilation, and air conditioning (HVAC) systems in conditioning the outside air make up as well as re-circulated air. iii DEDICATION This thesis is dedicated to my mother and my father. They gave me the encouragement I needed to continue on to graduate school and expand my horizon of possibilities. iv ACKNOWLEDGMENTS First and foremost, I would like to express a sincere gratitude to Professor Shelly Miller for her huge support and assistance throughout the entire length of this study. Secondly, I thank Professor John Zhai for his guidance and assistance on this project. I thank the American Society of Heating, Refrigeration and Air-Conditioning Engineers (ASHRAE) for supporting and funding this project. I express gratitude to the students who helped with the test setup and acquirement of data for this project: Joshua Droege, Iain Elliott and Nick Ortiz. I am extremely grateful to Professor Darin Toohey for all the time he volunteered to help make this project happen as well as providing most of the experimental instrumentation. I would like to acknowledge both Denver Public Schools and the Boulder Valley Public School District for finding appropriate school buildings for the tests to take place in, particularly directors Dale Hobbs and Morgan R. Deane, Jr. for giving the initial consent. I thank Bruce Gandrud and Kurt Sendelweck at Particle Metrics, Inc. for all the time they took to keep the testing instrumentation running in great condition and ready to use on our schedule. Finally, a warm thanks to all the facilities managers, building managers, and those who worked inside the test locations who allowed us to carry out so many days of testing in their buildings. v TABLE OF CONTENTS Signatories..................................................................................................................... ii Abstract ........................................................................................................................ iii Dedication .................................................................................................................... iv Acknowledgments......................................................................................................... v Contents ....................................................................................................................... vi List of Tables ............................................................................................................... ix List of Figures ............................................................................................................... x Chapter I: Introduction .................................................................................................. 1 Background ............................................................................................................. 1 Objective ................................................................................................................. 4 Chapter II: Methods ...................................................................................................... 6 Instrument Description............................................................................................ 6 Aerosol Mass Spectrometer .............................................................................. 6 Ultra High Sensitivity Aerosol Spectrometer ................................................... 8 Li-Cor CO2/ H2O Analyzer ............................................................................... 9 Testing Sites .......................................................................................................... 10 Denver School of the Arts............................................................................... 11 Grant Street Building ...................................................................................... 18 Eisenhower Elementary School ...................................................................... 25 Integrated Teaching and Learning Laboratory ............................................... 33 Experimental Setup ............................................................................................... 40 Primary Data Collection ................................................................................. 40 vi Secondary Data Collection ............................................................................. 44 Chapter III: Results and Discussion ............................................................................ 48 Building Characteristics ........................................................................................ 48 Instrument Characteristics .................................................................................... 49 Line Losses ........................................................................................................... 51 Weather ................................................................................................................. 54 Types of Data Collected........................................................................................ 57 Identification and Classification ........................................................................... 63 General Trends ................................................................................................ 64 Seasonal, Weekly, and Daily Trends .............................................................. 77 Correlations ........................................................................................................... 83 Lag Times ....................................................................................................... 85 Linear-Fit Slopes ............................................................................................. 92 Ammonium Nitrate Infiltration ....................................................................... 97 Chapter IV: Conclusions and Further Research ........................................................ 104 Conclusions ......................................................................................................... 104 Recommendations for Further Research ............................................................. 108 References ................................................................................................................. 110 Appendix A: Testing Site Floor Plans .........................Error! Bookmark not defined. Appendix B: Outdoor Hour-Averaged AMS Chemical Data Plots .. Error! Bookmark not defined. Appendix C: Indoor and Outdoor UHSAS Mass Concentrations Plots .............. Error! Bookmark not defined. vii Appendix D: Indoor and Outdoor UHSAS Number Concentration Plots ........... Error! Bookmark not defined. Appendix E: Indoor and Outdoor PM0.7 Number Plots ............. Error! Bookmark not defined. Appendix F: Indoor and Outdoor CO2 Plots ................Error! Bookmark not defined. Appendix G: Lag Time and Variability Tables ...........Error! Bookmark not defined. Appendix H: Indoor/Outdoor Ratio and Correlation Slope Tables .. Error! Bookmark not defined. viii LIST OF TABLES Number Page 1. Summary of HVAC system in each building. ................................................ 39 2. Summary of filters used in each building. ...................................................... 39 3. Summary of testing dates. ............................................................................... 44 4. Summary of air exchange rates. ...................................................................... 49 5. Seasonal average temperatures (°F) at each building. Standard deviations given in parenthesis......................................................................................... 54 6. Seasonal average PM2.5 values (μg/m3) at each building. Standard deviations given in parenthesis......................................................................................... 54 ix LIST OF FIGURES Number Page 1. Predicted total respiratory deposition based on ICRP deposition model. ......... 2 2. Aerodyne aerosol mass spectrometer (AMS). .................................................. 7 3. Particle Metrics Ultra High Sensitivity Aerosol Spectrometer (UHSAS). ....... 9 4. Li-Cor CO2 / H2O Analyzer. ........................................................................... 10 5. Denver School of the Arts, Denver CO. ......................................................... 11 6. Map to Denver School of the Arts. ................................................................. 12 7. Hallway near sampling location at Denver School of the Arts. ...................... 13 8. Eastern end of hallway and sampling location at Denver School of the Arts. 14 9. Floor plans of sampling area at Denver School of the Arts. Sample locations denoted by X’s. ............................................................................................... 15 10. Science room at Denver School of the Arts. ................................................... 16 11. Science room and sampling location at Denver School of the Arts. .............. 16 12. Outside sample location at Denver School of the Arts. .................................. 17 13. 1640 Grant Street, Denver CO. ....................................................................... 18 14. Map to 1640 Grant Street. ............................................................................... 19 15. Third floor office space and sampling locations at the Grant Street building. 21 16. Third floor sampling location and window location at the Grant Street building. .......................................................................................................... 21 17. Floor plans of third floor in the Grant Street building. Sampling locations (new on left, and old on bottom) denoted by X’s. .......................................... 22 18. Copy room/ mailroom and outdoor location at the Grant Street building. ..... 23 19. Outside location at the Grant Street building. ................................................. 23 20. Air intake and exhaust at the Grant Street building. ....................................... 24 x 21. Eisenhower Elementary School, Boulder CO. ................................................ 25 22. Map to Eisenhower Elementary School. ......................................................... 26 23. Music room at Eisenhower Elementary School. ............................................. 27 24. Teachers’ lounge at Eisenhower Elementary School...................................... 27 25. Sampling location in the music room at Eisenhower Elementary. ................. 28 26. Sampling location in the teachers’ lounge at Eisenhower Elementary. .......... 29 27. Outside sampling location at Eisenhower Elementary School. ...................... 30 28. Floor plans at Eisenhower Elementary School. Sample locations denoted by X’s. .................................................................................................................. 31 29. HVAC rooms and air intakes at Eisenhower Elementary School................... 32 30. Integrated Teaching and Learning Laboratory, Boulder CO. ......................... 33 31. Map to Integrated Teaching and Learning Laboratory at CU......................... 34 32. Inside sampling locations at the ITL Laboratory. ........................................... 35 33. Stairwell sampling location at the ITL Laboratory. ........................................ 36 34. Floor plans and indoor sampling locations at the ITL Laboratory. ................ 37 35. Schematic of Experimental Setup. .................................................................. 41 36. Testing setup showing all instruments and solenoid switching valves. .......... 42 37. Calibration equipment for the AMS, used to generate size-selected monodisperse aerosols. ................................................................................... 43 38. Screenshot of CO2 Analyzer. .......................................................................... 45 39. Air exchange rate plot for Eisenhower Elementary School at night. .............. 48 40. Differences in apparent diameters between AMS, UHSAS and DMA. ......... 50 41. Theoretical particle losses due to deposition for steady, laminar flow through 50-ft of tubing. ................................................................................................ 51 42. Average number concentrations during line loss experiment. ........................ 52 43. Line losses and standard deviations through sample tube configuration. ....... 53 xi 44. Comparison of obtained UHSAS PM0.7 to CDPHE PM2.5 mass concentrations for Eisenhower Elementary School. ............................................................... 56 45. Comparison of obtained UHSAS PM0.7 and AMS PM2.0 to CDPHE PM2.5 mass concentrations for the ITL Laboratory. .................................................. 56 46. Comparison of obtained UHSAS PM0.7 to CDPHE PM2.5 mass concentrations for the Grant Street building. .......................................................................... 57 47. Comparison of obtained UHSAS PM0.7 and AMS PM2.0 and CDPHE PM2.5 mass concentrations for Denver School of the Arts........................................ 57 48. UHSAS indoor (top) and outdoor (bottom) number concentrations as a function of time and particle diameter at the ITL Laboratory during spring 2006................................................................................................................. 59 49. UHSAS indoor (top) and outdoor (bottom) mass concentration as a function of time and particle diameter at the ITL Laboratory during spring 2006. ...... 60 50. AMS outdoor mass concentration as a function of time and particle diameter at the ITL Laboratory during spring 2006. ..................................................... 61 51. AMS outdoor mass concentration as a function of time at the ITL Laboratory during spring 2006. ......................................................................................... 61 52. Hour-Averaged AMS outdoor mass concentration as a function of time at the ITL Laboratory during spring 2006. ............................................................... 62 53. Averaged diameter at the ITL Laboratory in spring 2006 for AMS (left) and UHSAS (right). ............................................................................................... 63 54. Averaged number concentrations for the entire sampling year, as a function of optical diameter (from UHSAS data). ............................................................ 64 55. Averaged mass concentrations and indoor/outdoor ratio for the entire sampling year, as a function of optical diameter (from UHSAS data). .......... 65 56. Filter efficiency for individual single-fiber mechanisms and total efficiency. 66 57. Averaged mass concentration distributions and indoor/outdoor ratios for the entire sampling year at the ITL Laboratory (left) and the Grant building (right). ............................................................................................................. 67 58. Averaged number concentrations and indoor/outdoor ratios in Boulder and Denver. ............................................................................................................ 68 59. Number concentrations at the Grant Street building in the spring. ................. 68 xii 60. CO2 versus scheduled occupancy for the ITL Laboratory during the fall season. ............................................................................................................. 69 61. Indoor/outdoor ratio increasing with fresh air intake percentage. .................. 70 62. Indoor/outdoor ratios by mass and number, as a function of fresh air intake for Denver School of the Arts............................................................................... 71 63. Indoor/outdoor ratios by mass and number, as a function of fresh air intake for the ITL Laboratory. ......................................................................................... 71 64. Indoor/outdoor ratios by mass and number, as a function of fresh air intake for the Grant Street building. ................................................................................ 72 65. Indoor/outdoor ratios by mass and number, as a function of fresh air intake for Eisenhower Elementary School. ..................................................................... 72 66. Indoor/outdoor mass ratio as a function of wind speed for all data. ............... 73 67. Indoor/outdoor number ratio as a function of wind speed for all data. ........... 74 68. Indoor/outdoor mass ratio as a function of wind speed for Denver School of the Arts only. ................................................................................................... 74 69. Indoor/outdoor mass ratio as a function of wind speed for the summer only. 75 70. Indoor/outdoor ratio as a function of outdoor concentration for all data. ....... 76 71. Indoor number concentration as a function of excess indoor carbon dioxide for all data. ............................................................................................................ 76 72. Indoor/outdoor ratio as a function of excess indoor carbon dioxide for all data. ......................................................................................................................... 77 73. Ambient PM number distributions (top) and indoor/outdoor ratios (bottom) averaged by season. ........................................................................................ 78 74. Ambient PM mass distribution averaged over each season. ........................... 79 75. Indoor/outdoor ratios averaged over all datasets, split by period of week. .... 80 76. Indoor/outdoor ratios averaged over all DSA datasets, split by period of the week. ............................................................................................................... 81 77. Indoor/outdoor ratios averaged over all Grant Street building datasets, split by period of the week. .......................................................................................... 81 78. Indoor/outdoor ratios averaged over all Eisenhower Elementary datasets, split by period of the week. ..................................................................................... 82 xiii 79. Indoor/outdoor ratios averaged over all ITL Laboratory datasets, split by period of the week. .......................................................................................... 82 80. Indoor/outdoor ratios averaged over all ITL Laboratory datasets, separated by HVAC usage. .................................................................................................. 83 81. Indoor-outdoor ultrafine number correlation for weekday Grant building data in the fall. ........................................................................................................ 85 82. Time series autocorrelation plot for the ITL Laboratory during spring.......... 86 83. Ultrafine indoor-outdoor number concentration correlations without lag time shift (left) and with 36-minute lag time shift (right) for winter Grant Street building data.................................................................................................... 87 84. Average lag times. .......................................................................................... 88 85. Average constancy of the lag times. ............................................................... 89 86. Lag times at the ITL Laboratory by HVAC usage. ........................................ 90 87. Variability in lag times at the ITL Laboratory by HVAC usage. ................... 90 88. Average lag times at each building during the weekday daytime. ................. 91 89. Average lag times at each building during the weekend nighttime. ............... 91 90. Correlation slopes of entire sample year by period of week. .......................... 92 91. Correlation slopes of every building’s weekday daytime data combined, separated by season. ........................................................................................ 93 92. Correlation slopes of each building’s weekday daytime fine PM data per season. ............................................................................................................. 94 93. Correlation slopes for ITL Laboratory only, as a function of HVAC usage. . 94 94. Indoor/outdoor ratios for the ITL Laboratory, as a function of HVAC usage.95 95. Pearson’s correlation coefficients averaged by period of the week. ............... 96 96. Pearson’s correlation coefficients for the ITL Laboratory as a function of HVAC usage. .................................................................................................. 97 97. Ambient chemical makeup for Denver School of the Arts in the winter. ....... 98 98. Indoor-outdoor fine PM correlation difference for a high ammonium nitrate event at Denver School of the Arts in the winter. ........................................... 99 xiv 99. Indoor-outdoor ultrafine PM correlation difference for a high ammonium nitrate event at Denver School of the Arts in the winter............................... 100 100. Average AMS size distribution for Denver School of the Arts in the winter. ....................................................................................................................... 100 101. Indoor-outdoor ultrafine PM correlation difference for a high ammonium nitrate event at Denver School of the Arts in the fall. .................................. 101 102. Indoor-outdoor fine PM correlation difference for a high ammonium nitrate event at the ITL Laboratory in the spring. .................................................... 102 103. Indoor-outdoor ultrafine PM correlation difference for a high ammonium nitrate event at the ITL Laboratory in the spring. ......................................... 102 104. Indoor versus outdoor mass concentrations by chemical species for Denver School of the Arts during winter. .................................................................. 103 xv CHAPTER I INTRODUCTION Background Surprising increases in illness and death during several air pollution episodes during the mid twentieth century, such as the 1952 London smog, demonstrated that air pollution was hazardous to public health. Subsequently, many governments finally introduced legislation to reduce pollutant emissions into the atmosphere and it was thought that air pollution was no longer a public health problem (Holland et al. 1979). More recently, in the early 1990s, many epidemiologic studies suggested that air pollution, even at the lower ambient air concentrations that had been achieved with regulations and control technology, was still associated with cardiopulmonary disease and mortality (Samet et al. 2000; Schwartz 1994); especially the fine combustion-source pollution most commonly found near heavy traffic areas (Pope 2000). Many studies report that adverse health effects are most strongly correlated with the small particles that have diameters smaller than 2.5 microns (PM2.5), termed fine particles, originating from ambient emissions mainly derived from fossil fuel combustion. It has been suggested that the ultra-fine particles (PM0.1), or those with diameters smaller than 0.1 microns, can penetrate deep into the alveolar region and provoke inflammation, rousing acute respiratory illness in susceptible individuals, as well as increasing blood coagulability (Seaton et al. 1995). Figure 1 illustrates the predicted total and regional respiratory deposition for light exercise, based on the 1 International Commission on Radiological Protection (Hinds 1999). This shows the increased alveolar deposition in the ultrafine range. Although it has been investigated whether or not the toxicity of particulate matter is more dependent on its chemical composition or on its size, little evidence supports one idea over the other (Harrison and Yin 2000). Figure 1: Predicted total respiratory deposition based on ICRP deposition model. Because people spend upwards of 85% of their time indoors, it is widely recognized that a significant portion of total personal exposure to ambient particulate matter occurs in indoor environments including home, schools, offices and transportation environments. Data indicates that residential buildings can act as sinks for air contaminants (Chao 2001), suggesting the likeliness of infiltration. Thatcher and Layton (1995) showed that the building shell is ineffective against removing infiltrating particles. They found the particle penetration factor to be unity (Thatcher and Layton 1995), although other studies indicate a value slightly less than one (Long 2 et al. 2001; Mosley et al. 2001; Vette et al. 2001). Long et al. (2001) demonstrated that the penetration efficiency is reduced in homes that are kept tight with low air exchange rates. Moreover, the indoor/outdoor PM concentration ratio is reduced by the use of a central air cleaning system (Colome et al. 1992; Thornburg et al. 2001), mostly due to impaction on the filter and deposition to the ducting. In addition to generalizations on how ambient particulate matter infiltrates into indoor environments, many studies have measured actual indoor and outdoor concentrations in residential environments. Most of these studies have traditionally examined particulate matter with diameter less than 10 microns (PM10) or PM2.5 (eg., Colome et al. 1992; Fischer et al. 2000; Long and Sarnat 2004). Other studies simply used a passive sampler to collect a mass concentration (Clayton et al. 1993; Spengler et al. 1981), which overlooks the significance of the ultra-fine particulate matter as well as time-resolved information. These studies have shown that indoor particulate matter concentrations are consistently lower than ambient concentrations without the presence of indoor sources. Studies have shown minimal importance of particle resuspension on the indoor submicron PM concentrations. Lefcoe and Inculet (1975) found that indoor activity had little effect on particles less than 1 micron and Thatcher and Layton (1995) found that submicron particles are essentially nonsuspendable under typical activities found in residences. It has been observed that indoor concentrations are strongly correlated with outdoor levels of PM2.5 (Long and Sarnat 2004) and even more so when the air exchange rate is high (Phillips et al. 1993). The indoor/outdoor relation as a function 3 of particle diameter and chemical composition in the submicron range, however, remains unknown. Additionally, certain aspects of mechanically ventilated buildings have yet to be examined, such as how the amount of fresh air drawn into the building through the ventilation system affects the indoor/outdoor ratio. To better understand the increased health risk of ultra-fine particles, more data is required on indooroutdoor concentrations. These data are needed to design appropriate ventilation systems and control strategies that can minimize exposure to ultra-fine particulate matter. Objective The objective of this research is to provide details on the size and chemical speciation of ultrafine and fine particulate matter at two locations, inside and outside of one school and one office building at each location. The study will provide data that can be used to compare and correlate indoor and outdoor particulate matter concentrations. Particulate matter will be classified by size as established in American Society of Heating, Refrigeration and Air-Conditioning Engineers (ASHRAE) Standard 52.2 and the major components will be chemically identified. All four seasons of the year will be studied as well as differences between day and night and differences between weekdays and weekends. First and foremost, this study will provide important data needed to understand exposure and health risks associated with inhalation of ultrafine and fine particulate matter. In addition, the information provided by this study can improve 4 understanding of filtration requirements in buildings. The correlation of particulate concentrations between indoors and outdoors can be used to evaluate the performance of heating, ventilation, and air-conditioning (HVAC) systems in conditioning the outside air make up as well as re-circulated air. 5 CHAPTER II METHODS Instrument Description Our understanding of aerosol behavior and processes has always been limited by the particle detection instrumentation available. Recently, new technology has been created in response to the need for instrumentation that can provide real-time analysis of size resolved aerosol, mass, and chemical composition. The aerosol mass spectrometer manufactured by Aerodyne Research, Inc. (Billerica, Massachusetts) for example, has the capability of providing quantitative information on particle size distributions as a function of chemical composition in real time (Jayne et al. 2000). As a result, monitoring fine particulate matter mass concentrations and chemical compositions with high time resolution is made possible in indoor/outdoor air quality studies. Aerosol Mass Spectrometer The Aerodyne aerosol mass spectrometer (AMS) is a fast and reliable instrument for continuous monitoring of fine aerosol non-refractory chemical composition and size-resolved particle mass distributions as a function of particle composition (Figure 2). With this instrument, particles are sampled from ambient conditions into a high vacuum chamber through an aerosol-focusing inlet. This inlet forms a particle beam that is chopped at a fixed frequency. Particles passing though the chopper are impinged onto a resistively heated filament (temperature ~1500 °C) where the 6 volatile and semi-volatile components are flash vaporized. The vapors from the particle are ionized by electron impact and the ions are detected by a standard quadrupole mass spectrometer, yielding molecular composition information. For the particular AMS used in this experiment, the sample flow rate is restricted by a critical orifice inlet with a diameter of 100 microns, providing a flow of 85 cm3 min-1. Figure 2: Aerodyne aerosol mass spectrometer (AMS). In addition to generating a particle beam, the aerosol-focusing inlet allows a high fraction of particles to be analyzed, ranging from 30 nm to ~2.5 microns in diameter for non-refractory components. The measured particle collection efficiency is approximately unity for most particle sizes, dropping off due to impaction for larger particles and due to weak focusing of smaller particles (Jayne et al. 2000). Chopping 7 the particle beam allows the aerodynamic particle size to be determined by the aerosol time-of-flight to the vaporization filament. In tests of the aerosol time-offlight response, the particle velocity was found to be proportional to the aerodynamic diameter for a variety of different aerosol compositions (Jayne et al. 2000). More detailed information about the mechanics of the AMS is given by Jayne et al. (2000). Ultra High Sensitivity Aerosol Spectrometer The Particle Metrics, Inc. (Boulder, Colorado) Ultra High Sensitivity Aerosol Spectrometer (UHSAS) is an optical-scattering laser-based aerosol particle spectrometer system for accurately and precisely sizing particles in the range of 55 nm to 1.0 micron in diameter (Figure 3). It uses fully user-specified size binning of up to 100 channels anywhere within its size range. The laser is a semiconductordiode-pumped Nd3+:YLF solid-state laser with an intracavity power of ~1 kW. The standing wave laser mode is perpendicular to the flow of particles; the light is linearly polarized with the electric field vector parallel to the flow of particles. Particle scatter is collected in a direction perpendicular to both the particle flow and the laser standing wave. As particles traverse the laser mode, they scatter light into the detection system. The amount of light scattered is a strong function of the particle size. There are two pairs of collecting optics: one pair images onto an Avalanche photodiode for detecting the smallest particles, and the other pair images onto a lowgain PIN photodiode for detection of the upper size range of the instrument. Each detector is amplified in a current-to-voltage stage, which feeds the analog electronics system. 8 Figure 3: Particle Metrics Ultra High Sensitivity Aerosol Spectrometer (UHSAS). The main advantage of the UHSAS is its high resolution. The time resolution can be set to as fast as one second and the diameter resolution as low as 1.6 nm. For this study, the time interval was chosen to be 10 seconds, meaning all the particles triggers are counted in a histogram for 10 seconds and subsequently recorded. The 100 size bins were set on a logarithmic scale over its full range, 55 nm to 1 micron. The flow rate was set to 24 cm3 min-1. Li-Cor CO2 / H2O Analyzer The Li-Cor (Lincoln, Nebraska) LI-7000 CO2/ H2O Gas Analyzer is a high performance, dual cell, differential gas analyzer (Figure 4). It uses a dichroic beam splitter and two separate detectors to measure infrared absorption by CO2 and H2O in the same gas stream. The instrument software provides continuous output of the absolute concentration in the sample cell as well as the differential concentration when a reference cell concentration is known (a zero CO2 scrubber in this study). This instrument offers a 1% accuracy of CO2 and time resolution can be set as high as 50 Hz. The time resolution of the CO2 analyzer for this study was set to 0.2 Hz and 9 the flow was 1000 cm3 min -1 , as it was the free factor for ensuring isokinetic sampling at the point where the air flow rate changes in the sample tubes (discussed below in the experimental setup section). Figure 4: Li-Cor CO2 / H2O Analyzer. Testing Sites Two areas of the United States in Colorado were chosen for this study. The first area is Denver Colorado, which is a heavy metropolitan area with major high motor traffic areas and industrial facilities. The area just north of the Denver study locations is Commerce City, an Environmental Justice area that received over 200,000 pounds of legal hazardous emissions in 2004 (TRI data). This area also typically records the highest PM concentrations in metro Denver (EPA data). Emissions result from the activities of some of the 2,500 businesses/industries located in and around the area, including the interstates (I-25, I-70, and I-270), Colorado highways, industries located in adjoining unincorporated Adams County, and numerous diesel-trucking depots. The second area chosen for study is Boulder Colorado, which is a light metropolitan area with very few heavy industries and only one major interstate highway and one Colorado highway, and is 30 miles northwest of Denver on the 10 easternmost foothills of the Rocky Mountains. One office building and one school was chosen to study for each area. Denver School of the Arts In Denver Colorado, the school that was examined for this study is Denver School of Arts (Figure 5), located at 7111 Montview Blvd., Denver, CO 80220 (Figure 6). This school is unique in the Denver Public School District in that it serves both middle school (6th through 8th grade) and high school (9th through 12th grade) students. The enrollment at this public school was 881 students at the time of this study. This school was chosen because of its proximity to major air pollution sources in Commerce City as well as the accessibility of HVAC information. Figure 5: Denver School of the Arts, Denver CO. 11 Figure 6: Map to Denver School of the Arts. Completed in February 2004, Denver School of the Arts was built around an existing university level fine arts center located on a 7.3-acre site in Denver’s Park Hill Neighborhood. This 115,000-sq-ft addition enveloped 90 percent of the existing building with new construction. The state-of-the-art design featured a concert hall, theatre, dance studios, and many individual practice rooms. The new construction also added 40 new classrooms, a library, and 2 acting studios. This unique remodeling project provided an opportunity to deal with specific technical issues related to acoustics, sound systems, performance lighting, theatrical rigging and drapery systems in the various performance spaces. The building is two-story brickwalled structure with tile flooring in most areas. The air sampling done at this building was on the second floor at the easternmost end of the main hall, above the cafeteria and administrative offices, in part of the newer construction. This hallway has its second story walkway such that the second 12 floor is open to the first floor over a railing (Figure 7), and comes to an end with a staircase (Figure 8). The furthest room to this eastern end on the second story is the mechanical room, a large open space where the HVAC units are located. This was where the sampling equipment was stored during air sampling. This mechanical room has two external walls (north and east) and is adjacent to a classroom that serves as the science room. The hallway has several 1-ft diameter diffusers spanning its entire length, and is open to the front entryway of the building that has six doubledoors. These doors are sometimes propped open. A schematic of the floor plans in this area are illustrated in Figure 9. Figure 7: Hallway near sampling location at Denver School of the Arts. 13 Figure 8: Eastern end of hallway and sampling location at Denver School of the Arts. 14 X Science room Mechanical room X Hallway X Figure 9: Floor plans of sampling area at Denver School of the Arts. Sample locations denoted by X’s. The science room is a 1025-sq-ft room used by twenty to thirty students during school hours (Figure 10 and Figure 11). The room has six 1-sq-ft air diffusers and one air return vent and three opening windows totaling 4-ft tall and 8-ft wide. This tile-floored room has fifteen workbench-style tables with at least 30 stools, two large marker boards, and roughly twenty five computers placed on cabinets which boarder the room. The room has two internal doors, a false ceiling, and a separate joining storage room for long-term storage of any chemicals used. 15 Figure 10: Science room at Denver School of the Arts. Figure 11: Science room and sampling location at Denver School of the Arts. 16 Two sampling tubes were routed from the instrumentation in the mechanical room through the firewall into the false ceiling above the science room. One tube was dropped into the science room (Figure 11) and the other went beyond to the hallway, also dropped through the false ceiling. The inside lines hung about two feet from the ceiling at 10-30 degrees from the downward vertical. The third line was routed through the air intake vent to the outside of the building. The outside line rested about two feet away from the edge of the northern exterior wall, approximately 20-ft above the ground (Figure 12). Figure 12: Outside sample location at Denver School of the Arts. The HVAC systems in this building are driven by variable speed drive fans that control the static pressure throughout the system. There are 23 variable air volume boxes on the HVAC unit that supplies air to the sample locations of this study. The HVAC units for this building utilize patent vinyl-framed, 4” pleated polyester filters 17 with 35% efficiency, which are replaced every three months. [more information on the HVAC controller will be included soon! -Nick] Grant Street Building The Denver office building that was examined for this study (Figure 13) is located at 1640 Grant Street, Denver, CO 80203 (Figure 14). This is a three-story multipurpose building, and will hitherto be referred to as the Grant Street building. The Grant Street building is approximately 5 miles west of Denver School of Arts, in the downtown area but not far from I-25 and I-70. Figure 13: 1640 Grant Street, Denver CO. 18 Figure 14: Map to 1640 Grant Street. The three-story brick building, built in 1960, is home to an environmental law firm whose offices utilized the entire third floor. The first and second stories of the Grant building consisted of offices including franchising, accounting and bookkeeping, as well as a copying services company on the first floor. The third story, containing roughly fifteen offices with doors, was carpeted throughout. Each office with an external wall also contained at least one opening window measuring roughly 4-sq-ft. Each room had at least a single 1-sq-ft air diffuser and a return air vent. The entire space was covered with a false ceiling. As this is a law firm, there is typically one employee assigned to a room, and their occupancy varies but can exceed 40 hours per week. For the first two sampling campaigns (summer and fall), the indoor sampling at the Grant building was in two separate office rooms on the third floor. Each of these rooms measured approximately 116-sq-ft and 13.5-ft tall with a single 1-sq-ft air 19 diffuser and return air vent. The outside sampling line was slipped through a north exterior window, which was afterwards sealed with duct tape to avoid infiltration. The sampling equipment was located in an office room separate from those being sampled. Due to a rearrangement of offices halfway through the study, this space was no longer available for the sampling equipment during the winter and spring sampling campaigns, and thus the sampling was moved to slightly different indoor and outdoor locations, still on the third floor. The new indoor sampling space was one large room, open to the hallways (Figure 15). The room is approximately 250-sq-ft and has two 1-sq-ft air diffusers and one return air vent. This room had a couple of work desks and was the storage space of numerous files and books both in cabinets and on shelves. There were two opening windows on the north side of this room (Figure 16) which overlook the 10 – 15 space parking lot. The windows measured approximately 3-ft by 3-ft each. Both indoor lines were sampling this room, one hung from the false ceiling on each side of the room. All sampling locations, new and old, are illustrated in Figure 17. 20 Figure 15: Third floor office space and sampling locations at the Grant Street building. Figure 16: Third floor sampling location and window location at the Grant Street building. 21 X X X N X X X Figure 17: Floor plans of third floor in the Grant Street building. Sampling locations (new on left, and old on bottom) denoted by X’s. The equipment was located in the adjacent copy/mailing room (Figure 18) and the outdoor line was slipped through an east external window of the copy room, roughly 20-ft above the ground level (Figure 19). 22 Figure 18: Copy room/ mailroom and outdoor location at the Grant Street building. Figure 19: Outside location at the Grant Street building. This office building utilizes a dual-duct HVAC system that was built in 1961. It uses 2” pleated filters, which are replaced every three months. The chiller has a cooling capacity of 100 tons (1,200,000 BTUH), and the boiler has a capacity of 60 23 tons (720,000 BTUH). The fixed-speed fan is scheduled to run Monday through Friday from 5am – 8pm and on Saturdays and Sundays from 6am – 6pm. The fresh air damper is controlled to maintain a supply air temperature of about 52°F, while using a minimum of 10% fresh air. Overnight when the HVAC system is not in use, a perimeter heat radiation system turns on if the outside air temperature drops below 40°F. The HVAC system and all its components are located in the basement. The air intake for this building is on the north side of the building on the second floor with the exhaust approximately eight feet higher (Figure 20). This air intake faces the building’s parking lot. Figure 20: Air intake and exhaust at the Grant Street building. 24 Eisenhower Elementary School In Boulder Colorado, the school in which air sampling took place was Eisenhower Elementary School (Figure 21), located at 1220 Eisenhower Dr., Boulder, CO 80303 (Figure 22). This building is in a quite neighborhood, placing it no closer than 1000ft to the nearest major road and roughly one mile east of the I-36. This location was chosen as well for the HVAC accessibility. Figure 21: Eisenhower Elementary School, Boulder CO. 25 Figure 22: Map to Eisenhower Elementary School. This 46,000-sq-ft brick building was built in 1971. Enrollment at this K – 5 public school was 438 at the time period of this study. The hallways of this school were floored with tile, whereas the classrooms were carpeted. The entire building was covered with false ceiling and many of the classrooms were separated by partitions. At the southwestern corner of the school lies a classroom which was used for the music room (Figure 23) and just to the east of this was the teacher’s lounge (Figure 24). These two rooms were the indoor locations chosen for this building. The music room is occupied at various times throughout the day for regular classes as well as music classes. There were times throughout the day when the room was unoccupied. The teachers’ lounge was seldom occupied, but the teachers would occasionally make brief visits to this room. 26 Figure 23: Music room at Eisenhower Elementary School. Figure 24: Teachers’ lounge at Eisenhower Elementary School. 27 The music room was approximately 800-sq-ft and 9-ft tall. It had one seldomused external door and one internal door but no windows. There was a chalkboard in the room, few desks, and roughly 15 plastic chairs. The sample tube was hung from the false ceiling at one end of the room, near the entry door (Figure 25). The teacher’s lounge was about 390-sq-ft and 8-ft tall with one sealed external window and two internal doors that typically remain open. This room contained a couch made of faux leather and two upholstered sofas, as well as a few wooden tables. There was also a microwave, refrigerator, and toaster in this room. The sampling tube was just inside the entry door (Figure 26). Both rooms had three 1-sq-ft air diffusers and one return air vent; both rooms were carpeted. The cafeteria kitchen was within 30-ft of each room. These two rooms were on separate ventilation zones and separate air handling units. Figure 25: Sampling location in the music room at Eisenhower Elementary. 28 Figure 26: Sampling location in the teachers’ lounge at Eisenhower Elementary. Across the hallway from the music room, adjacent to the kitchen, was the boiler room and janitor’s storage room. The sampling equipment was stored in the janitor’s room and the outside line was routed through the boiler room and out the vents to the outside (Figure 27). This placed the outside sampling line near the west exterior wall, where the bus-loading zone is located. A schematic of the sample locations is illustrated in Figure 28. The rest of the school’s floor plan are given in Appendix A. 29 Figure 27: Outside sampling location at Eisenhower Elementary School. 30 Boiler Room Janitor’s Room X Kitchen X Music Room X Teachers’ Lounge N Figure 28: Floor plans at Eisenhower Elementary School. Sample locations denoted by X’s. The four HVAC units are on the roof, as well as the air intakes (Figure 29) and chillers. The air handling units are constant volume and utilized 2” pleated filters. 31 Figure 29: HVAC rooms and air intakes at Eisenhower Elementary School. The Eisenhower Elementary School’s HVAC schedule is controlled by a Direct Digital Control (DDC) system. The units run continuously during occupied hours set by the timelock of the DDC controller. When any of the units are off (i.e. unoccupied periods), the mixing dampers are in the normal position, which means the outside air and relief air dampers are closed, while the return air dampers are open. Also, the fan and coil pumps become disabled at this time. When the outside air temperature is above 75 ºF or below 52 ºF, the outside air damper is set in the minimum position. The building is heated based on the lowest space temperature. When the zone with the lowest temperature drops to 55 ºF, the coil pump is enabled, the zone dampers opened to 100%, the hot deck opened, and the fans are then enabled. This occurs until the low zone temperature reaches 57 ºF, at this time the fan and coil pump become disabled. The night purge occurs when the highest temperature exceeds 2 ºF above the outside air temperature. At this time the outside air and relief air dampers are opened, the return air damper closes, all of the zone dampers open 100% to the cold deck, and the fan is enabled. As soon as the high zone temperature drops to 2 ºF below the 32 occupied setpoint, the fan becomes disabled, and the dampers are set back to the normal position. Integrated Teaching and Learning Laboratory The other building examined in Boulder was the Integrated Teaching and Learning (ITL) Laboratory (Figure 30). This is a three-story, 34,000-sq-ft learning facility located at 1045 Regent Dr., Boulder, CO 80309 on the University of Colorado campus adjoining the Engineering Center (Figure 31). This is approximately 1.5 miles west of Eisenhower Elementary, 400 feet from I-36. Figure 30: Integrated Teaching and Learning Laboratory, Boulder CO. Completed in 1997, the concrete-walled ITL Laboratory is a shared classroom facility for the six departments of CU-Boulder's College of Engineering and Applied Science. This state-of-the-art facility features design studios, an active learning center, and extensive computer network integrating a wealth of experimental equipment throughout the two large, open laboratory plazas, team workrooms, and manufacturing centers including a machine shop and three project fabrication facilities. 33 Figure 31: Map to Integrated Teaching and Learning Laboratory at CU. This facility has as many as 150 students scheduled to be in the laboratory plazas and classrooms at one time for various classes. There are also several students who use the facility on their own time either for studying, using the computers/modules, or working on projects. The floors in this majority of the facility were tiled, though the study rooms and classrooms were carpeted. For this study, the main level laboratory plaza was the location of one indoor line (Figure 32). This plaza consists of 15 – 20 computer workstations and is open to the high ceiling approximately 40 feet above. The floor is tiled and there are several sealed windows in this plaza. The fabrication facilities and machine shop were not located on this level. One indoor sampling line was hung by the yellow support beam seen in Figure 32, near a corner of the laboratory space. The other indoor line was routed to the stairwell between the first and second floors of the building, near the study rooms and open to the main laboratory plaza (Figure 33). The outdoor line was 34 routed to the HVAC intake plenum on the roof, sampling air just before it entered the air handling unit. All sampling equipment was kept in the mechanical room (location of the HVAC unit). The floor plans for this area are illustrated in Figure 34. More detailed floor plans are supplied in Appendix A. Figure 32: Inside sampling locations at the ITL Laboratory. 35 Figure 33: Stairwell sampling location at the ITL Laboratory. 36 N Classrooms Laboratory plaza X Mechanical room Study rooms X Figure 34: Floor plans and indoor sampling locations at the ITL Laboratory. The air handling unit serving the ITL Laboratory was a variable air volume type with a supply volume capacity of 48,300 CFM. The unit runs on a pre-programmed Direct Digital Control system, the operating schedule depending on the occupancy schedule. This air handling unit is unique in that it utilizes a direct evaporative cooling section in addition to the indirect cooling coil. The evaporative cooling is used because the humidity in Colorado is typically low, making this type of cooling more cost efficient. However, when the humidity in the air increases, the indirect cooling coil is automatically activated. The supply fan is 54.7 HP fan with a speed of 884 rpm. The return fan is 30.3 HP with a speed of 725 rpm. 37 The ITL Laboratory has two main operating schedules. The first schedule takes place when the building is occupied during the school year. The second takes place when the building is not as heavily occupied, during the summer months. When the building is occupied, the discharge air temperature is set based on the outside air temperature (OAT) according to two schedules. The first schedule is when the OAT exceeds 55 ºF, the discharge air temperature will be 55 ºF. The second schedule is when the OAT falls below 20 ºF, the discharge temperature will be 65 ºF. During the occupied period, the supply fan speed is controlled to maintain a 1.0” WG static pressure in the supply duct. The return fan speed is controlled to maintain a 0.1” WG in the return air plenum. The exhaust air damper position is adjusted to maintain a static pressure of 0.05” WG. The outside air dampers are controlled by the DDC to maintain a minimum fresh air flow of 8,700 CFM. When the building is unoccupied, the fans are de-energized and the outside and exhaust air dampers are closed, while at the same time the steam coil control valve is opened to the coil. The radiation heating valves are controlled to maintain a temperature of 65 ºF in each zone. If this temperature drops below 63 ºF, the air handling unit cycles on to maintain the zone temperature using supply air at a maximum temperature of 85 ºF. During this study, the HVAC system was turned on most weekdays between 6AM and 7AM and turned off at 6PM in the summer and 10PM for all other seasons. There was one winter Monday when the HVAC was not operating. The weekend schedule 38 during this study was 11AM to 3PM. Sometimes the HVAC turned on as early as 10:20AM. A summary of the HVAC system used in each building is given below in Table 1. The filter summary of each building follows in Table 2. Table 1: Summary of HVAC system in each building. Building AHU type Supply Capacity (CFM) Variable Air Volume 32,000 Dual-duct NA Eisenhower Elementary Constant Volume 16,450 Integrated Teaching and Learning Laboratory Variable Air Volume 48,300 Denver School of Arts Grant building Table 2: Summary of filters used in each building. Building Denver School of the Arts Grant building Filter type 4” pleated polyester pads 2” pleated, pinch frame Rated Avg. Efficiency* MERV rating** 35 % NA 30-35 % 8 2” pleated, high 25-30 % 7 capacity Integrated Teaching and 2” pleated, high 30-35 % 8 Learning Laboratory capacity * Rated Average Efficiency as described by ASHRAE standard 52.1 ** Minimum Efficiency Reporting Value (MERV) as described by ASHRAE standard 52.2. Eisenhower Elementary 39 Experimental Setup In deciding an appropriate place inside each building to sample, it was important to consider the instrumentation. The AMS, being a kilowatt instrument, dissipates a lot of heat while running and therefore needs to sample from a wide-open space. Without sufficient ventilation and cooling in the room, the turbopumps tend to overheat and stop running. In addition to this, the AMS should be stored away from both children and public, as the slightest tampering could cause malfunction in the instrument. A tighter requirement still, the equipment had to be placed within 50 feet of indoor sampling locations as well as outside access to route the sampling lines. Unfortunately, the small rooms at Eisenhower Elementary School and the Grant Street building did not allow for a suitable sampling location. It was decided to sample without the AMS at these locations, omitting the AMS data and thus the chemical speciation data from one building in both Boulder and Denver. Primary Data Collection For each of the buildings, three sample lines were routed such that two indoor locations and one outdoor location could be reached from an appropriate equipment storage location. Each line was a 50-foot, ½-inch nominal diameter copper tube. A schematic of the experimental setup is given in Figure 35. The sampling inlet was split using a solenoid-switching valve that switched between the three lines every 4 minutes. Air was drawn through the 50-foot tubes with a large pump, drawing approximately 12 L min-1 through each tube. This ensured updated concentration readings directly following a line-switch. Before the air was drawn through this large 40 pump, a smaller inlet with a much smaller flow (approximately 1.0 L min-1) sampled this air. This was where the switching valves were located. Finally, after the three sampling lines merged to a single one, it was split again to the three sampling instruments via ¼-inch copper tubing. Figure 35: Schematic of Experimental Setup. Where the flow changes before the large pump, the flow rates had to be adjusted to ensure isokinetic sampling (the air speed through the tubes must equal the velocity of the sampling inlet), although these submicron particles are hardly affected by anisokinetic sampling. The variables available to adjust these flow rates were the wide range of possible flow through the CO2 analyzer as well as a flow restrictor 41 before the large pump. The copper tubes were grounded to avoid electrostatic forces from charged particles. A photograph of this setup is given in Figure 36. Figure 36: Testing setup showing all instruments and solenoid switching valves. Data was collected for about a week at a time (4 – 8 days), ensuring the collection of two 24-hour periods during a weekday and a weekend at each test site. The AMS was size calibrated prior to usage at each test site and the ionization efficiency was calibrated a few times throughout that week. Figure 37 shows the calibration equipment required for the AMS. To generate ammonium nitrate particles for size calibration, a pump first pushed air through a filter, then into a TSI, Inc. Model 3076 Constant Output Atomizer (TSI, Inc. 2001) in combination with a TSI, Inc. Model 3062 Diffusion Dryer (TSI, Inc. 2001). The polydisperse aerosols were sized to a 42 mobility diameter of 350 nm using a TSI, Inc. Model 3080 Electrostatic Classifier with a Model 3081 Long Differential Mobility Analyzer (DMA) (TSI, Inc. 1999). The generated aerosol was transported via ¼ inch copper tubing directly into the AMS inlet. Figure 37: Calibration equipment for the AMS, used to generate size-selected monodisperse aerosols. The UHSAS was set to a sample flow rate of 24 cm3 min-1 at standard conditions. One hundred logarithmic bins were sampled between 55 nm and 1 micron. The number histogram was counted and saved every ten seconds on the UHSAS to avoid its upper count limit. The AMS, having to scan through several masses for size determination, requires more time before saving. This instrument was set to save every four minutes, alternating 5 seconds on the mass spectrum (MS) mode and 25 seconds on the time-of-flight (TOF) mode. 43 A summary of the testing dates are listed in Table 3. Two times during the summer, the UHSAS and AMS did not collect representative data. These were due to flow and leak problems. These instances called for a second week of sampling to obtain the missing data. Without enough summer time left to sample again, the data during the summer at Eisenhower Elementary School was deemed unusable because of a leak in the sample lines. Table 3: Summary of testing dates. Summer 2005 Fall 2005 Winter 2006 Spring 2006 ITL Laboratory Jul. 27-Aug. 2 Aug. 3 – 10* Oct. 19 – 26 Jan. 12 – 18 Apr. 5 – 12 Denver School of the Arts Aug. 11 – 17 Aug. 17 – 22* Oct. 26 – Nov. 4 Jan. 19 – 25 Apr. 12 – 19 Grant building Aug. 22 – 29 Nov. 30 – Dec. 5 Jan. 25 – 30 Apr. 19 – 25 Eisenhower Elementary Aug. 29 – Sep. 6** Nov. 21 – 30 Jan. 30 – Feb. 6 Apr. 25 – May 2 * The second sampling week was for UHSAS data, which does not overlap with the AMS data. ** The data from this sample week was flawed by a leak in the lines. Secondary Data Collection In addition to sampling particulate matter, the amount of CO2 in the indoor and outdoor locations was also measured. The CO2 provided in-situ visual information on the switching status as well as leak indications (Figure 38). An indication of leaking would be a visual influence from breathing near the sampling equipment. The CO2 data provided a good indication of when occupants were present in the sampling 44 locations. When the building was unoccupied, the indoor CO2 concentration levels dropped to the outdoor background levels. . Figure 38: Screenshot of CO2 Analyzer. Before each sampling campaign at each building, the air exchange rates (AER) were assessed for each sampling room using a tracer gas test protocol with CO2. The CO2 tracer gas method provides only the AER for the actual room in which sampling takes place and cannot be used in large areas or hallways. This is done by releasing CO2 into the room from a high-pressure CO2 tank, allowing up to 5000 ppm into the room. The monitor shown in Figure 38 proved useful for this. The solenoid switching valves were manually overridden to monitor only the room with the elevated CO2. Once the desired concentration was reached, the CO2 tank was closed and the natural decay was recorded until background levels (~450 ppm) were reached. This method was not possible at ITL Laboratory, its indoor volume being too large for increasing the CO2. The AER can also found using the ratio of the volumetric flow rate of air into the space to the interior volume of the space. The supply flow into the sample rooms was obtained using a bolometer capture hood, measuring the volumetric air flow from the 45 supply diffusers. Since the supply flow is being used rather than fresh air, this is referred to as the space air exchange rate. Three different methods were used to find the percentage of fresh air in the supply air. At Denver School of the Arts, a direct readout of the fresh air damper position was logged by the HVAC department of Denver Public Schools. The ITL Laboratory has an online sensor system called Building as a Learning Tool (BLT). This BLT system allows for acquiring data about the HVAC system, including the flow rates of the supply, fresh, return and exhaust air streams. The percentage of fresh air in the supply air in this building was found simply by dividing the fresh air flow rate by that of the supply air. The BLT system is also the source of supply flow for the second method of finding the air exchange rate at the ITL Laboratory, since the bolometer measurements would have been difficult in this large space. The remaining two buildings do not have this kind of online computer technology, and thus a different method was required. Temperature sensors were placed in the ducting to record the temperature of the outside air, return air, and mixed air. The sensors used were portable, battery-powered temperature data loggers. Equation 1 was used to find the fresh air make-up, based on the principle that the mixed air will have a temperature that is either closer to the outside air or return air, depending on which is being used for supply air in greater quantity. Since the two rooms at Eisenhower Elementary School are on different zones, two sets of temperature loggers had to be used in the separate air handling units. 46 FreshAir % Tmized Treturn *100% Toutside Treturn (1) This method for finding the fresh air intake percentage is not as reliable a method as the direct readout of the fresh air damper position obtained at Denver School of the Arts, nor is it as reliable as the air flow rates obtained by the BLT system at the ITL Laboratory. The temperature method is inherently flawed by the fact that the temperatures are influenced by external factors such as the sun directly radiating on the outside sensors, or the time it takes for the temperatures to adjust when the damper position changes. However, with careful examination of the temperature data, the general opening and closing of the fresh air damper can be assumed. 47 CHAPTER III RESULTS & DISCUSSION Building Characteristics During each sampling week, the air exchange rates were found using a CO2 tracer gas method. This could not be done in the ITL Laboratory, since it is far too large a space for this method. Two separate sample rooms were chosen for Eisenhower Elementary School, so the air exchange rate was found for each room. At Denver School of the Arts, the air exchange rate could be found in the science room, but not in the hallway, where it was open to the rest of the building. To find the AER, the natural logarithm of the CO2 was plotted against the time in hours. The slope of the linear fit through this data is the AER in air changes per hour (ACH). An example of this plot is given in Figure 39. Figure 39: Air exchange rate plot for Eisenhower Elementary School at night. 48 The results for the space air exchange rates using the full range of the tracer gas protocol method and the supply air flow method are given below in Table 4. Most of the air exchange rates are in the typical design range. The teacher’s lounge at Eisenhower Elementary was exceedingly high, possibly due to a design flow rate for cigarette smoking, as the building was built in 1971. Each air exchange rate test was conducted during the day when the HVAC was running, except for the single overnight run at Eisenhower Elementary. This was done in the music room with the purposes of establishing a typical nighttime air exchange rate. Table 4: Summary of air exchange rates. Air Exchange Rate (ACH) Air Supply Flow (ACFM) Interior Volume (ft3) Denver School of the Arts 1.3 – 3.4 517 9,230 Eisenhower Elementary (teacher’s lounge) 16 – 28 1,443 3,120 Eisenhower Elementary (music room) 2.9 – 8.8 1,057 7,200 Eisenhower Elementary (music room at night) 0.114 - - 3.3 – 5.5 184 2,000 4.0* 20,400 306,000 Sample Location Grant building Integrated Teaching and Learning Laboratory * This value is based on a rough estimate of the interior volume. Instrument Characteristics The data from the two instruments differ in that the UHSAS counts the number of particle light-reflection pulses it sees in each size bin whereas the AMS measures the 49 ion bursts and is scaled up to a mass. The two can be compared if the UHSAS data is converted from a number to a mass concentration assuming a density and spherical particles. Ambient air is comprised of a variety of particles, the average submicron particle densities have been reported to vary from 1.2 to 1.8 g cm-3 (Morawska et al. 1999) with an average dry density of 1.48 g cm-3 (Stein et al. 1994). Since the AMS data in this study show mostly organics, which have a density of ~1.2 g cm-3, the atmospheric density in this study was assumed to have a slightly larger density to compensate for the nitrate and sulfate compounds, at 1.3 g cm-3. An experiment investigating the differences in these instruments was carried out to better understand the discrepancies. It was found that the UHSAS optical diameter is approximately 75% of the AMS aerodynamic vacuum diameter, but the optical diameter was very close to the mobility diameter found from the electrostatic classifier/ differential mobility analyzer (Figure 40). The AMS measures more mass during a real test because its particle cutoff size is higher than that of the UHSAS. Figure 40: Differences in apparent diameters between AMS, UHSAS and DMA. 50 Line Losses Line losses can be expected due to diffusion of aerosol particles to the walls of a tube as they flow through it. However, due to the small deposition parameter (D*L/Q < 0.002 for all particle diameters examined in this study) the losses by deposition to the walls from this steady, fully laminar flow through a tube are very small (Hinds 1999, pg. 163). The losses by diffusion were estimated to be insignificant, with losses of less than 1.5 % through the 50-feet of tubing (Figure 41). More losses can be expected from inertial impaction around the bends of the particle transport tubes, as well as through the solenoid switching valves configuration and through the ninety-degree bends used to split the lines to the instrument inlets. Figure 41: Theoretical particle losses due to deposition for steady, laminar flow through 50-ft of tubing. An experiment was conducted to determine the actual amount of line losses as a function of particle diameter. The three 50-ft lines were set up outside such that one line was coiled up to sample next to the UHSAS. The switching valves manually 51 kept this one line open. The UHSAS was set with the same settings as used during normal sampling, and the CO2 analyzer was used as well for consistency. After five minutes of sampling, the switching valves and 50-foot tubes were disconnected and the UHSAS sampled directly from its own outlet for another five minutes. This was alternated back and forth until each sampling scheme gathered eight 5-minute subsets, totaling 80 minutes of data. The average number concentrations during this test are given in Figure 42. Figure 42: Average number concentrations during line loss experiment. The losses plotted in Figure 43 summarize the average particle concentration losses as a function of size from each of the eight subsets. The average losses across the size range were around 20 – 30%. The standard deviation from the average is also shown in this plot, and it can be seen that the larger aerosols had a much higher variability in the line losses. This can be attributed to the lower concentrations at the 52 larger particle sizes. This size had a small enough concentration for the variability during these 80 minutes to become significant and even dominating in the line loss uncertainty. The data from 700 nm – 1 micron appeared flawed in every dataset due to binning the wrong size particles, and so only the data from 55 – 700 nm will be used in this study. Figure 43: Line losses and standard deviations through sample tube configuration. The total loss from the entire sample line configuration was 22.4 ± 7.1% by number and 23.5 ± 8.7% by mass. However since the focus of this study is on the difference between indoor and outdoor PM concentration values rather than the absolute magnitudes, this line-loss error does not need to be implemented into the data; the losses effect the values from all three sample lines equally. 53 Weather Meteorological data was obtained from nearby meteorological stations. There was a station in Boulder located at 2120 Marine St. There were two used in Denver; the station closest to the Grant building is located at 2105 Broadway St. and the station closest to Denver School of the Arts was actually in Commerce City, located at 7101 Birch St. The meteorological data collected for this study were temperature (Table 5) and PM2.5 (Table 6), as well as relative humidity, ozone, SO2, NO2, wind speed and direction. The PM2.5 instruments used at the meteorological stations were Rupprecht and Patashnick Co., Inc. Series 1400a Tapered Element Oscillating Microbalance (TEOM), with the Series 8500 Filter Dynamic Measurement System (FDMS). Table 5: Seasonal average temperatures (°F) at each building. Standard deviations given in parenthesis. Building Summer 2005 Fall 2005 Winter 2006 Spring 2006 ITL 72.1 (9.6) 49.7 (8.7) 40.5 (8.5) 55.6 (10.9) Laboratory Denver School 69.0 (9.7) 52.3 (10.5) 32.0 (9.8) 61.3 (12.4) of the Arts Grant building 73.9 (9.1) 30.3 (9.9) 39.2 (8.5) 50.5 (14.6) Eisenhower 71.2 (10.6) 41.5 (10.8) 39.8 (7.5) 53.7 (11.1) Elementary Table 6: Seasonal average PM2.5 values (μg/m3) at each building. Standard deviations given in parenthesis. Building Summer 2005 Fall 2005 Winter 2006 Spring 2006 ITL 5.18 (2.38) 1.28 (1.91) 1.16 (2.38) 11.85 (4.70) Laboratory Denver School 5.96 (3.54) 13.85 (13.99) 10.83 (11.78) 10.04 (3.25) of the Arts Grant building 7.64 (3.04) 12.22 (11.24) 14.89 (6.07) 13.75 (4.16) 54 Eisenhower Elementary 6.97 (3.32) 2.30 (4.14) 1.49 (2.69) 4.31 (5.47) The CDPHE (Colorado Department of Public Health and Environment) PM2.5 data was compared with the outdoor UHSAS data obtained in this study. The PM data recorded by the UHSAS includes particles with diameters as large as 0.7 microns, and the heavier particles that make up the PM2.5 were not seen. As a result, the UHSAS mass tends to be much less than that of the meteorological station, though the general trends appear to be similar. The AMS was underestimating the mass as well, with its upper limit near two microns. Additionally, both the UHSAS and AMS were losing some 20% of the total mass by particle deposition to the sample tubes (see Line Losses Section). There is also some difference in ambient concentrations in different local areas, with smaller concentrations next to the building walls. Therefore, this comparison is only a means of validating the general rising and falling of ambient particulate mass concentration values and thus, the appropriateness of the choice of weather station used. Eisenhower Elementary was the location where the UHSAS ambient data was most similar to the CDPHE PM2.5 (Figure 44). The next best meteorological data was that taken near the ITL Laboratory, with the exception of springtime being different by a factor of about five (Figure 45). The two Denver buildings showed values less than the meteorological stations’ mass concentration data (Figure 46 and Figure 47), suggesting that much of the ambient particulate matter had diameters between 1.0 and 2.5 μm. 55 Figure 44: Comparison of obtained UHSAS PM0.7 to CDPHE PM2.5 mass concentrations for Eisenhower Elementary School. Figure 45: Comparison of obtained UHSAS PM0.7 and AMS PM2.0 to CDPHE PM2.5 mass concentrations for the ITL Laboratory. 56 Figure 46: Comparison of obtained UHSAS PM0.7 to CDPHE PM2.5 mass concentrations for the Grant Street building. Figure 47: Comparison of obtained UHSAS PM0.7 and AMS PM2.0 and CDPHE PM2.5 mass concentrations for Denver School of the Arts. Types of Data Collected The data from the aerosol mass spectrometer are written as IGOR Text files, and these were analyzed using the scientific graphing and data analysis program IGOR 57 Pro, developed by WaveMetrics, Inc. (Lake Oswego, OR). The data was analyzed using an AMS analysis toolkit developed by James Allan at Manchester University. The AMS analysis toolkit (version 1.37) was modified to meet the goals of this study. An example of the basic plots that can be made with the UHSAS data is given in Figure 48 (spring 2006 sampling campaign at the ITL Laboratory in Boulder). Time is on the x-axis and particle diameter on the y-axis; the number concentration is illustrated by the color of the image. The two inside locations were averaged together and plotted on the top. The outdoor data is plotted on the bottom, and there is a visual correlation between the two: the indoor number concentration appears to follow that of outdoor, but with lower concentrations. The rest of these plots, one for each sampling week, can be found in Appendix D. The week of sampling at Eisenhower Elementary School in the summer was left out of all the data analysis since it was apparent that the three lines were leaking and thus the data is misrepresentative. 58 Figure 48: UHSAS indoor (top) and outdoor (bottom) number concentrations as a function of time and particle diameter at the ITL Laboratory during spring 2006. The previous plot redrawn as a mass concentration is illustrated in Figure 49. The mass concentration shows different information, such as the large-diameter event that takes place on April 8, 2006. Although the mass plots look like they contain more useful information, the ultrafine particulates have virtually no mass and therefore the number concentrations will be used heavily in this study for correlations and comparisons. Appendix C contains all the mass concentration plots. 59 Figure 49: UHSAS indoor (top) and outdoor (bottom) mass concentration as a function of time and particle diameter at the ITL Laboratory during spring 2006. The outdoor AMS data for the same week is presented in Figure 50. This data has smoothing applied in both the time and diameter directions for clearer viewing. The data looks similar to that of the UHSAS. The aerodynamic vacuum diameters of major events on this plot are larger than the optical diameters in Figure 49. Something to note about the AMS data is the “holes” in the plot where the instrument recorded negative mass values. These values were due to inevitable noise fluctuations of the AMS signals and were not plotted. The fluctuations are better seen on the next graph, which is the total integrated mass concentration as a function of time (Figure 51). On this plot, the major chemical constituents can be seen in addition to the total mass. 60 Figure 50: AMS outdoor mass concentration as a function of time and particle diameter at the ITL Laboratory during spring 2006. Figure 51: AMS outdoor mass concentration as a function of time at the ITL Laboratory during spring 2006. The main advantage to gain by using the AMS is the chemical speciation of the ambient mass concentrations. Figure 51 shows not only the total mass that can be compared directly to the UHSAS data, but also the chemical makeup. There is an event on Saturday, April 8 for example, that shows nitrate levels higher than on any other day. It can be seen that the ammonium peaks here as well. This powerful capability will prove useful in finding a correlation between indoor/outdoor concentration levels as a function of chemical species. chemical speciation plots is included in Appendix B. 61 Each of these weekly Averaging the data in one-hour blocks eliminates the noise fluctuations in the AMS data, providing easier data analysis (Figure 52). Figure 52: Hour-Averaged AMS outdoor mass concentration as a function of time at the ITL Laboratory during spring 2006. The data from both instruments can also be manipulated to find the most prominent diameters by mass. Averaging the outside data over the entire week yields the results in Figure 53. The shapes of these two plots are very similar, but the diameter is shifted as described above (by about 75%), due to differences in opticalversus aerodynamic vacuum diameter measurements. The AMS plot (Figure 53, left) shows that the average organic particle diameter is smaller than those comprised of nitrate or sulphate. 62 Figure 53: Averaged diameter at the ITL Laboratory in spring 2006 for AMS (left) and UHSAS (right). Identification and Classification The data found over this yearlong period represent only sixteen 4 – 8 day sample periods. Although this may seem like a lot of data, it is only a fraction of the entire year in which sampling took place. With that noted, it must be reminded to the reader that the data presented in this paper are a small snapshot of everyday indoor/outdoor air quality values and can only be taken as general ideas or trends. It was assumed that with four buildings being examined over four seasons that enough data would be available to make general statements about the differences between daytime and nighttime PM values as well as seasonal and daily trends. However, a week of data collection can have great variations to any other week, with the vast number of external factors influencing air quality. The standard deviations of the PM concentrations are large, and therefore omitted from the plots for clarity. The following data is presented in such a way as to generalize the results in the most likely fashion. 63 General Trends Over all sixteen datasets, it is apparent that the outdoor particulate matter concentrations were generally higher than those of indoor were, with the exception of indoor source occurrences. Figure 54 shows the overall indoor and outdoor mass concentration averages from the entire dataset. The highest number concentration was at 55 nm and drops with increasing diameter. Figure 54: Averaged number concentrations for the entire sampling year, as a function of optical diameter (from UHSAS data). Figure 55 shows the same data as a mass concentration, as well as the indoor/outdoor ratio. The outdoor concentrations were typically 50% larger than indoor, a ratio ranging from 0.45 – 0.6 across the entire size range. The peak mass occurred at 186 nm for the outdoor average and 180 nm for indoors. The averaged indoor/outdoor ratio had a local maximum at a diameter of 165 nm and a minimum at 397 nm. 64 Figure 55: Averaged mass concentrations and indoor/outdoor ratio for the entire sampling year, as a function of optical diameter (from UHSAS data). The other thing to note from this plot is that the ratio has a local maximum at 165 nm and a minimum at a particle size of 400 nm. The increase at 165 nm can be explained by typical filter efficiencies dropping near size, the size that is too large for diffusion to be effective and too small for impaction or interception to be effective (Hinds 1999, pg. 198). These mechanisms along with the total filter efficiency are illustrated in Figure 56. 65 Figure 56: Filter efficiency for individual single-fiber mechanisms and total efficiency. Each building had its own unique indoor/outdoor trend. For example, the indoor/outdoor ratio at the ITL Laboratory had a prominent local minimum at particle diameters of 300 – 400 nm, averaged over all four seasons (Figure 57, left). This results from the specific combination of filters, HVAC ducting, and even the evaporative cooling pads that this building uses. No other buildings have this characteristic with such prominence. Denver School of the Arts had a similar dip in the indoor/outdoor ratio but only during the fall and winter. The Grant Street building had a completely different ratio distribution, as seen in (Figure 57, right). This building’s indoor/outdoor ratio had a local maximum at 186 nm and there was no local minimum where the ITL Laboratory had one. 66 Figure 57: Averaged mass concentration distributions and indoor/outdoor ratios for the entire sampling year at the ITL Laboratory (left) and the Grant building (right). As expected, the outside particulate matter concentrations in Denver were slightly higher than those in Boulder. This is even truer of the indoor particle concentrations and especially for diameters less than 150 nm. As the number and mass distributions appear very similar, the difference between Denver and Boulder is better seen as total number concentrations (Figure 58). Although the indoor/outdoor ratios were similar, those of the buildings in Boulder averaged mostly between 0.4 and 0.5 across the entire size range, whereas the average ratio at the Denver buildings was about one tenth higher. 67 Figure 58: Averaged number concentrations and indoor/outdoor ratios in Boulder and Denver. The indoor mass concentration, integrated across the entire size range, followed that of the outdoors. This can be seen in any dataset, such as in the following time series plot of the Grant Street building number concentration in the spring (Figure 59). All of the other number concentration plots can be found in Appendix E. Figure 59: Number concentrations at the Grant Street building in the spring. The CO2 inside the building was a strong function of occupancy (Figure 60); allowing assumptions to be made on how many people were in the sampling room when an occupancy schedule could not be obtained. This should be an indication of 68 when the indoor particle resuspension rate was likely to be highest, increasing the indoor/outdoor ratio. It should be noted that the obtained occupancy schedule plotted in the following figure reflects only the classroom enrollment, not the people who used “drop-in” hours in the laboratory. There are many people who “drop-in” to use the ITL Laboratory throughout the entire day and evening. All of the remaining CO2 plots are included in Appendix F. Figure 60: CO2 versus scheduled occupancy for the ITL Laboratory during the fall season. During only a few sampling campaigns, the indoor/outdoor ratio was found to be strong function of the fresh air intake into the building. In other words, when the HVAC unit brought in more air from the outside, the indoor/outdoor ratio often increased and approached a value of unity. Conversely, the indoor/outdoor ratio sometimes dropped when the HVAC unit was recirculating the inside air or was turned off. Although dependence can be seen, there are always other factors influencing the indoor/outdoor ratio. An example of when the fresh air percentage 69 appears to have had a large influence was seen in the summer at the ITL Laboratory (Figure 61). Figure 61: Indoor/outdoor ratio increasing with fresh air intake percentage. This relation between the fresh air intake and the indoor/outdoor ratio, however, was only a weak correlation. When the entire dataset is plotted and a linear fit is made, it can be seen that the ratio tended to be higher when more fresh air entered the buildings, but the correlation coefficients were very small. Denver School of the Arts (Figure 62) and the ITL Laboratory (Figure 63) had the highest dependencies of fresh air intake on the ratio. Denver School of the Arts had the highest correlation. This relationship at the Grant Street building (Figure 64) and Eisenhower Elementary (Figure 65) showed low dependencies as well as low correlations with the data. A higher correlation means that the indoor/outdoor PM ratio is a stronger function of the fresh air percentage. Since the correlations for the four buildings are about 0.6, 0.3, 0.2 and 0.0, it can be concluded that overall, the indoor/outdoor ratio is only weakly dependent on the fresh air intake. 70 Figure 62: Indoor/outdoor ratios by mass and number, as a function of fresh air intake for Denver School of the Arts. Figure 63: Indoor/outdoor ratios by mass and number, as a function of fresh air intake for the ITL Laboratory. 71 Figure 64: Indoor/outdoor ratios by mass and number, as a function of fresh air intake for the Grant Street building. Figure 65: Indoor/outdoor ratios by mass and number, as a function of fresh air intake for Eisenhower Elementary School. The only other parameter that showed some influence on the indoor/outdoor ratio was the wind speed. It was found that the mass ratio (Figure 66) correlates better than the number ratio (Figure 67). Because the number concentration is dominated 72 by the ultrafine particles and the mass concentration by the large fine particles, this result suggests that the wind has minimal effect on submicron particle resuspension. Denver School of the Arts had the highest relation between the two (Figure 68), followed by Eisenhower Elementary. The ITL Laboratory and Grant Street building had low correlations between indoor/outdoor ratios and wind speed. The summer showed the highest correlation (Figure 69), followed by fall and winter. The spring season had the lowest correlation between indoor/outdoor ratios and wind speed. Figure 66: Indoor/outdoor mass ratio as a function of wind speed for all data. 73 Figure 67: Indoor/outdoor number ratio as a function of wind speed for all data. Figure 68: Indoor/outdoor mass ratio as a function of wind speed for Denver School of the Arts only. 74 Figure 69: Indoor/outdoor mass ratio as a function of wind speed for the summer only. Other parameters that were investigated for indoor air quality dependence were the temperature, relative humidity, ozone, nitrogen dioxide, sulfur dioxide, and wind direction. None of these parameters showed any correlation with the indoor/outdoor PM ratios or the indoor PM concentrations. It was anticipated that higher outdoor concentrations would increase the indoor/outdoor ratio due to higher concentration gradients; and although it did increase the indoor concentration, the ratio was not influenced with any noticeable correlation (Figure 70). It was also anticipated that the excess indoor CO2 (an indication of indoor occupancy) might have an effect on either the indoor PM concentration (Figure 71) or the indoor/outdoor ratio (Figure 72) but again, there is no apparent correlation. This result agrees with the previous studies that found minimal submicron particle resuspension (Lefcoe and Inculet 1975; Thatcher and Layton 1995). The wind speed may therefore fit into this category of particle resuspension, as it too was a minimal influence on the smaller sized particles. 75 Figure 70: Indoor/outdoor ratio as a function of outdoor concentration for all data. Figure 71: Indoor number concentration as a function of excess indoor carbon dioxide for all data. 76 Figure 72: Indoor/outdoor ratio as a function of excess indoor carbon dioxide for all data. Seasonal, Weekly, and Daily Trends Air quality, both indoors and outdoors, is highly dependent on the season. Inversion layers can trap the particles and increase PM concentrations. It was found in this study that the fall had the highest outdoor number concentrations, followed by winter, spring then summer (Figure 73). The indoor/outdoor ratio was highest during the summer and spring. This could account for the opening of windows (for those buildings with opening windows), the propping open of doors, or more fresh air being brought inside during these seasons. The spring ratio was very similar to that of summer, and the fall ratio was similar to that of winter. 77 Figure 73: Ambient PM number distributions (top) and indoor/outdoor ratios (bottom) averaged by season. The plot of the outdoor mass distributions shows that the spring had the highest ambient mass peak, followed by the fall, winter then summer (Figure 74). This discrepancy between mass and number concentrations suggests that the particulate matter has a different effective bulk density throughout the year. The peak mass during the spring was at a particle diameter of approximately 272 nm, the fall peaked at 209 nm, and the other two seasons had the mass distribution peak just below 200 nm. 78 Figure 74: Ambient PM mass distribution averaged over each season. More analysis can be done after separating the data by day and night any by weekend and weekday. The reasons these might be different are building occupancy, differences between weekday and weekend traffic, and HVAC usage, among others. With these four mechanically ventilated buildings, a more appropriate way to break down the data is by times the HVAC system was usually turned on (weekday daytime), usually turned off (weekday and weekend nighttime) and when it was sometimes turned on (weekend daytime). The daytime period was chosen to be 6am – 8pm, when the HVAC system is usually running at all four buildings. The indoor/outdoor ratios looked similar for each period of the week, when averaged over all four seasons. The overall average ratio for the weekday-daytime was the highest for the larger size range of 500 – 700 nm; and the weekend-daytime had the highest ratio for the ultrafine size range (Figure 75). Overall, it can be said that there was not much difference in indoor/outdoor ratio for ultrafine and small fine 79 PM between the periods of the week. The following plots will aid in looking with more detail into the data, to check the validity of this conclusion. Figure 75: Indoor/outdoor ratios averaged over all datasets, split by period of week. The next four plots show the averaged period of the week data for each building. Figure 76 contains the DSA data, which shows very little difference between weekday and weekend data, but higher indoor/outdoor ratios for day than night. This result suggests that the HVAC usage at DSA brought in more ultrafine and small fine particulate matter. Figure 77 displays the Grant Street building ratio data, where the ratios were higher during the weekend than weekdays and higher during the nights than the days. This suggests that the HVAC usage decreased the amount of outdoor PM brought indoors, opposing the DSA results. 80 Figure 76: Indoor/outdoor ratios averaged over all DSA datasets, split by period of the week. Figure 77: Indoor/outdoor ratios averaged over all Grant Street building datasets, split by period of the week. The Eisenhower data showed a similar result to that of the Grant Street building, in that the nighttime indoor/outdoor ratio was higher for the ultrafine particles (Figure 78). The ITL Laboratory weekday ratio was clearly higher than the weekend, and the nighttime ratios were higher than the daytime ratios (Figure 79). This plot also shows that the weekday indoor/outdoor ratio had a sharp increase for particulates with diameters greater than 400 nm, possibly the effect of the particle resuspension range being approached. After examining each of the buildings individually, it holds that 81 there is not a clearly defined result regarding the difference between day and night or between weekday and weekend indoor/outdoor ratios. Figure 78: Indoor/outdoor ratios averaged over all Eisenhower Elementary datasets, split by period of the week. Figure 79: Indoor/outdoor ratios averaged over all ITL Laboratory datasets, split by period of the week. The data in Figure 79 can be compared to that in the next plot, which shows all the ITL Laboratory data separated by times the HVAC was in use or not (Figure 80). The ITL Laboratory was the only place where the exact times of HVAC usage were obtained and thus the most accurate dataset regarding the effects of ventilation. This 82 plot suggests that HVAC usage does bring more particulate matter into the building, with a smaller effect on the ultrafine particulate matter. Figure 80: Indoor/outdoor ratios averaged over all ITL Laboratory datasets, separated by HVAC usage. The increase in the indoor/outdoor ratio at diameters closer to 1 micron may have been due to resuspension from building occupants or other various indoor activities, as indicated by the more dramatic increase during weekday daytime periods (Figure 75). The weekday nights can have some resuspension influence from as few as one person in the building near the sample location. Correlations Indoor particulate matter concentrations (12-min averages) correlated linearly with outdoor values. A least-squares linear fit was applied to each dataset, providing the slope and the Pearson’s correlation coefficient. The slope of this linear fit differs from the previously discussed indoor/outdoor ratio in that the correlation slope is 83 more affected by the outliers of the dataset. As a result, the standard deviation of the indoor-outdoor correlation slope is much smaller than that of the ratio. Some outliers were so far from the regular trend of data that they were likely either the result of an indoor source or a misrepresentative spike in outdoor data values. These far outliers were removed prior to the linear fit. Analyzing the slope provides another method for understanding the relationship between indoor and outdoor concentrations. The Pearson’s correlation coefficient is a measure of how well the dataset can be described by this linear fit. In order to achieve the highest correlation coefficients from the least-squares linear fits, the data was broken down as before, into periods of the week that the HVAC system was typically scheduled to be on (weekday days), scheduled to be off (weekday and weekend nights), and sometimes turned on (weekend days). Since particulate matter infiltrates into each building uniquely and seasonal effects can be expected, data was broken down further by building and season to find individual correlations. To distinguish differences between particle sizes, two sets of the described data were studied: 55 – 100 nm (ultrafine) and 100 – 700 nm (fine). An example of one of these correlations (ultrafine weekday-daytime data during the fall at the Grant Street building) is given in Figure 81. 84 Figure 81: Indoor-outdoor ultrafine number correlation for weekday Grant building data in the fall. Lag Times The relationship between indoor PM concentrations and outdoor values did not always occur simultaneously, since there is some lag time to be expected for particle transport, ventilation, and infiltration. The lag times for indoor air quality following that of outdoor were found using time series autocorrelation. A linear fit was applied to each dataset for both fine and ultrafine number concentrations, and the Pearson correlation coefficient (Equation 2) was found. The lag times were applied to the data such that the outdoor event at time t corresponded to the indoor value at time t+∆t, where ∆t is the lag time, a 12-minute shift. The linear fit correlation coefficient was found for each twelve-minute shift (the lag time resolution for this study is limited by the full switching cycle of twelve minutes) up to 600 minutes. The maximum correlation coefficient corresponds to the most probable lag time between indoor and outdoor PM concentrations. An example of this autocorrelation is given 85 in Figure 82, the springtime ITL Laboratory data. The appropriate lag time shift was applied to all data used throughout the remainder of this paper. All the lag time data is presented in Appendix G. r (x n x)( y n y ) n (x n (2) x) 2 ( y n y ) 2 n Figure 82: Time series autocorrelation plot for the ITL Laboratory during spring. Applying the appropriate lag time shift to each of the data subsets greatly increased the correlation coefficients of indoor to outdoor PM concentrations. This is illustrated in Figure 83, the raw data for the ultrafine winter Grant Street building on the left and the 36-minute lag time shift applied to the data on the right. Including this shift increased the Pearson correlation coefficient from 0.759 to 0.853. The data points move in closer to the fit line. 86 Figure 83: Ultrafine indoor-outdoor number concentration correlations without lag time shift (left) and with 36-minute lag time shift (right) for winter Grant Street building data. The lag times were found for each building during each season, separated by weekday days, weekday nights, weekend days and weekend nights. Averaging over all seasons yields the data in Figure 84. The weekday days, when the HVAC was usually turned on, had the shortest lag time of about 11 minutes for both ultrafine and fine particulate matter. The lag time was generally shorter during the day than the night and shorter during the weekdays than the weekends. The nights had the biggest standard deviation in lag time, suggesting a large variability the driving forces for infiltration. 87 Figure 84: Average lag times. The lag times can differ quite a bit during the periods of the week; the sharpness of the time-series autocorrelation curve is an indication of the constancy in the lag times during those periods of the week. The sharpness can be measured by the full duration at half maximum (FDHM), shown in Figure 85. The constancy of the lag times for fine and ultrafine particles was most alike when the HVAC system was turned on (weekday daytime). This suggests that the usage of the HVAC system affects the ventilation lag times equally for both particle sizes. This observation, along with the comparable weekday daytime data from Figure 84 leads to the conclusion that the HVAC system brings in both particle sizes with a relatively short and constant lag time. 88 Figure 85: Average constancy of the lag times. The exact usage times of the HVAC system were only obtained at the ITL Laboratory. Whereas most of this study estimates HVAC system usage by probable times, knowing the exact times the HVAC system was turned on gives more information about its influence on indoor concentrations. Figure 86 shows the lag times averaged over periods the HVAC system was in use. The data in this plot agree with the period of the week data from Figure 84 and affirm that the HVAC system brings outdoor particles into the building consistently between 10 – 15 minutes with little difference between particle sizes. When the HVAC system is not in use, the ultrafine particles infiltrate the building faster than the larger particulate mass. Figure 87 shows the inconsistency of lag times when the HVAC system is not in use, by having a consistently larger FDHM of the autocorrelation curve. 89 Figure 86: Lag times at the ITL Laboratory by HVAC usage. Figure 87: Variability in lag times at the ITL Laboratory by HVAC usage. The following two figures show the difference in daytime and nighttime lag times separated by building. During the day when the HVAC system is in use, the longest lag time was for fine PM at Eisenhower Elementary (Figure 88). Denver School of the Arts had no apparent difference in fine and ultrafine PM, except that the fines had a more variable lag time. 90 Figure 88: Average lag times at each building during the weekday daytime. Figure 89 shows the average lag times at each building during the weekend nighttime, when the HCAC system was not in use. There was more difference in particle size for each building than during the daytime. This suggests that the ultrafine particles more easily penetrate the building during periods the HVAC is not in use. Figure 89: Average lag times at each building during the weekend nighttime. 91 Linear-Fit Slopes Once the appropriate lag time shifts were implemented into the data, the twelveminute indoor number concentrations were plotted against those of outdoor and a linear fit was applied. The slopes give an indication of how effectively outdoor particulate matter travels into the indoor environments, regardless of outside levels. As the value approaches unity, the indoor air quality matches that of outdoor. The values of this correlation slope, like the lag times, are dependent on the buildings’ particular HVAC and ducting schematic, the seasonal effects, the period of the week, and the particle size. Creating a linear fit to all the building’s indoor-outdoor PM concentrations and separating by the periods of the week yields the data in Figure 90. The ultrafine particles always had a higher correlation slope than the fine particles by about 20%. The daytime correlations slopes were higher than those of the nighttime were, but the weekdays and weekends have similar slopes. Figure 90: Correlation slopes of entire sample year by period of week. 92 Separating the weekday daytime data by season shows that there was smaller indoor-outdoor linear fit correlation slopes in the winter (Figure 91). One possible reason is that buildings are typically more sealed during the winter months, meaning the windows remain shut and doors are not kept open. A way to validate this theory is by separating the data in Figure 91 by building. The buildings with unsealed windows (Denver School of the Arts and Grant Street building) should show higher slopes during the warmer seasons. This was done in Figure 92, where only the fine particulate matter is shown. It can be seen from this figure that the correlation slope at Denver School of the Arts was higher than all other buildings during the summer, spring and fall, when the window of the sample location could likely have been opened. Although not quite as prominent, the Grant Street building data showed a similar result. The plot for ultrafines looks the same. As a result, open windows appear to have had a large contribution to the indoor air quality. Figure 91: Correlation slopes of every building’s weekday daytime data combined, separated by season. 93 Figure 92: Correlation slopes of each building’s weekday daytime fine PM data per season. In order to gain a better understanding of the effect of HVAC usage, it is best to examine the data in which the exact HVAC usage times were obtained. The ITL Laboratory correlation slopes for the linear fits of all “HVAC on” and “HVAC off” data are shown in Figure 93. It can be seen here that the ultrafine particles enter the building more effectively than larger sized particles, and both particle sizes have higher correlation slopes when the HVAC system is in use. Figure 93: Correlation slopes for ITL Laboratory only, as a function of HVAC usage. 94 At this point, it can be pointed out that this data analysis would not be possible with indoor/outdoor ratios. These plots show average ratios that are too similar to each other, with vary large standard deviations. Figure 94 shows the equivalent of the previous graph with ratios instead of slopes. The correlation slopes, being secondorder fits, prove to have much smaller standard deviations and values that are more useful for comparison. Figure 94: Indoor/outdoor ratios for the ITL Laboratory, as a function of HVAC usage. The correlation coefficient is a measure of how well the linear fit to the indooroutdoor data matches the actual data. In other words, a high correlation coefficient means that the relation between indoor and outdoor PM concentrations is well described by a linear function. The plot in Figure 95 shows that the ultrafine, on average, correlated better than the fine particulate matter. The weekdays had higher 95 correlation slopes than the weekends, but the day and night data were very comparable. Figure 95: Pearson’s correlation coefficients averaged by period of the week. Again, the effect of HVAC usage is best seen when examining the ITL Laboratory where the exact times on usage were obtained. This data is given in Figure 96. As noticed before, the ultrafine PM concentrations correlated better than those of the fine did. It is also apparent from this plot when the HVAC system was not in use, the larger indoor particles had a lower correlation with outdoor values. 96 Figure 96: Pearson’s correlation coefficients for the ITL Laboratory as a function of HVAC usage. Ammonium Nitrate Infiltration The Aerosol Mass Spectrometer allows us to know the chemical speciation of particulate matter. Although most of the ambient particulate matter throughout this study was organic, there were some occasional events where nitrate fragments dominated the atmospheric particulate matter. Figure 97 shows one of these sample periods, with a high nitrate event on 1/20/2006. The ammonium levels rise simultaneously with nitrates, suggesting that the makeup is ammonium nitrate particles. 97 Figure 97: Ambient chemical makeup for Denver School of the Arts in the winter. These particular events with high atmospheric nitrates were examined to see if there was a difference in ventilation and infiltration. Lunden et al. (2003) reported that measured indoor ammonium nitrate concentrations are lower than they would be based on penetration and deposition losses alone. They attributed the extra reduction to transformation indoors of ammonium nitrate into nitric acid and ammonia gases due to relative humidity and temperature changes (Lunden et al. 2003). The indoor surface area plays an important role in the equilibrium between the transformations. To see if the data from this study agree with this reduced ammonium nitrate phenomena, the periods with high nitrate levels were extracted from the rest of their sample week. The two subsets of data were plotted as indoor against outdoor number concentrations, and the correlation was examined. The AMS has some trouble analyzing ammonium particles; since they follow the trends of nitrate particles, only the high nitrate periods were examined to track ammonium nitrate events. 98 There were only a few sampling weeks with a high ammonium nitrate event; each of them showed similar results. Figure 98 shows the indoor-outdoor fine number correlations at Denver School of the Arts during the winter. The data during the ammonium nitrate event had a smaller linear-fit correlation slope, and the nitrate data fell below most of the other data on the curve. This means there was less infiltration when the PM was composed of a fair amount of ammonium nitrates. Figure 98: Indoor-outdoor fine PM correlation difference for a high ammonium nitrate event at Denver School of the Arts in the winter. Figure 99 shows a similar result but to a lesser degree. This is the same dataset, but only the ultrafine particulate matter is shown. Ammonium nitrate particles are typically larger than 100 nm (Figure 100), so it makes sense that the ammonium nitrate infiltration reduction was not so prominent in the ultrafine data. 99 Figure 99: Indoor-outdoor ultrafine PM correlation difference for a high ammonium nitrate event at Denver School of the Arts in the winter. Figure 100: Average AMS size distribution for Denver School of the Arts in the winter. The two other sampling weeks with a high ammonium nitrate event proved to have the same effect. Each ambient ammonium nitrate event corresponded to periods of reduced infiltration. The other time this occurred at Denver School of the Arts, in the fall, had a correlation difference that looks very similar to that of the winter (Figure 101). This similarity suggests that the indoor surface area may play an 100 important role in the transformation of ammonium nitrate into ammonia and nitric acid gases. Figure 101: Indoor-outdoor ultrafine PM correlation difference for a high ammonium nitrate event at Denver School of the Arts in the fall. The only observed ammonium nitrate event at the ITL Laboratory looks quite different (Figure 102). This may be due to a different relative humidity in the building since the HVAC system utilizes evaporative cooling or a different indoor surface area. The ultrafine particulate matter for this week had a reduction not quite as drastic, but still significant (Figure 103). 101 Figure 102: Indoor-outdoor fine PM correlation difference for a high ammonium nitrate event at the ITL Laboratory in the spring. Figure 103: Indoor-outdoor ultrafine PM correlation difference for a high ammonium nitrate event at the ITL Laboratory in the spring. Thus far, it is still ambiguous whether the reduced infiltration during a high ammonium nitrate event is indeed due to less ammonium nitrate particles and not a reduction other chemical species. Figure 104 verifies that the indoor-outdoor correlation is smaller for both ammonium and nitrate than for the organics. This is 102 the plot along with the linear fits for Denver School of the Arts during winter, when there was a fair amount of ammonium nitrate in the ambient air. Figure 104: Indoor versus outdoor mass concentrations by chemical species for Denver School of the Arts during winter. 103 CHAPTER IV CONCLUSIONS AND FURTHER RESEARCH Conclusions This study was carried out in order to find information regarding the ventilation and infiltration of outdoor fine and ultrafine particulate matter to typical indoor, mechanically ventilated environments. Using an aerosol mass spectrometer along with an Ultra High Sensitivity Aerosol Spectrometer, the total concentration, size distribution, and chemical composition of indoor and outdoor particulate matter were examined in search of a correlation between the two. Two mechanically ventilated buildings in the heavy metropolitan Denver, CO and two in the light metropolitan Boulder, CO were studied over all four seasons of the year to find seasonal, weekly, and daily variations in the indoor-outdoor correlation. The outdoor PM concentrations were, on average, higher than indoor values by 50% in the absence of indoor sources. The average indoor/outdoor ratio ranged from 0.45 – 0.6 for all particle diameters from 55 – 700 nm. The peak mass occurred at 186 nm for the outdoor average and 180 nm for indoor. The ratio peaks at a particle diameter of 165 nm and has a minimum at 397 nm. Each of the four buildings examined in this study showed a unique indoor/outdoor ratio size distribution due to the HVAC filters, ducting and control schedule, building specifics, and many other influencing factors. The two Denver buildings had higher particulate matter values than the two Boulder buildings, both outdoor and indoors. Additionally, the Denver average indoor/outdoor ratio was higher than the buildings in Boulder. So not only is 104 the air quality in Denver typically worse, but the indoor/outdoor ratios were worse in this study as well. The indoor/outdoor ratio was influenced by the fresh air intake, but only a slight connection between the two was seen. When the HVAC would bring more fresh air into the building rather than recirculated air, the indoor/outdoor ratio sometimes would increase. Against expectations, the ratio was not a strong function of building occupancy, when particle resuspension might be assumed. The only apparent effects of particle resuspension were for the particles with diameters approaching 1 micron, but these were only minimal. The wind speed showed some influence on the indoor/outdoor ratio as well. A higher wind speed seemed to increase the ratio but again, there was minimal effect the smaller sized particles. The indoor/outdoor ratio was also examined against meteorological data such as ambient temperature and ozone, but no influence was found with any of these data. The atmospheric air quality proved to be highly dependent on the season, being worse in the fall and best in the summer. The indoor/outdoor ratios were higher during the summer and spring; a contributing factor to this might be more opened windows and propped-open doors, or more fresh air ventilation. There is not a solid conclusion that can be drawn on the differences in the indoor/outdoor ratio between the day and night or between the weekdays and weekends. Each building had different results and neither one seems to have any reason for being a more reliable a result. However, it was shown that the indoor/outdoor ratio increased during the times when the HVAC system was in use. 105 When the indoor mass concentrations were plotted against the outdoor values, a linear relationship was found. The relationship correlates higher when separated by periods of the week when the HVAC system was usually turned on (weekday days), usually turned off (weekday and weekend nights) and sometimes turned on (weekend days). There was some lag time before high outdoor PM concentration events were seen indoors, and this was a varying amount of time dependent on the building, season, period of the week and particle size. The lag times during this study were as high as 480 minutes, with a twelve-minute resolution. There was an overall lag time of 42 minutes for ultrafine PM and 49 minutes for fine PM. One interesting result was that the nighttime average lag time was only about one hour for both particle sizes. Including the lag times in each dataset increased the indoor-outdoor linear fit correlation coefficients by a significant amount. The weekday daytime periods had the shortest lag time for this study. The lag times were typically shorter in the day than the night and shorter during the weekdays than the weekends. When the HVAC system was in use, both particle sizes had similar lag times. When the HVAC system was not in use, the ultrafine particles had consistently shorter lag times than the fines. The slopes of these indoor-outdoor PM linear fits represent the effectiveness of a building shell and its ventilation filtration to protect indoor environments from outdoor particulate matter. A higher slope indicates a less effective building shell and air filtration. The ultrafine particulate matter consistently had higher correlation slopes than the larger sized particles by nearly 20%. The daytime correlation slopes were higher than those of nighttime, and there was minimal difference between the 106 days of the week. The buildings had lower indoor-outdoor correlation slopes during the winter, when the windows and doors were more often closed. Examining specific HVAC usage times, it was found that ultrafine particles enter the building more effectively than larger sized particles, and both particle sizes have higher correlation slopes when the HVAC system is in use. It was also shown that these correlation slopes contain useful data that cannot be found in the indoor/outdoor ratios. The Pearson’s correlation coefficient is a measure of how well the indoor-outdoor linear fit describes the actual data. It was found that the ultrafines typically had a higher Pearson’s correlation coefficient. When the HVAC system was not in use, the fine particulate matter had lower correlation coefficients than the ultrafines, meaning that infiltration for larger sized particles did not fit a linear function as well as the ultrafines. The AMS allowed for chemical speciation of the particulate matter. Most of the time, the total PM mass was dominated by organics. There were a few events with high levels of ambient ammonium nitrate. These periods were studied to see if there were different ventilation characteristics. As shown in previous works (), the ammonium nitrate seemed to infiltrate much less than predicted by penetration and deposition losses alone. When the ammonium nitrates dominated the ambient mass concentration, the indoor-outdoor linear-fit slopes decreased. The linear-fit slopes did not decrease as dramatically for ultrafine PM as it did for fine PM. As the ammonium nitrate particles are not typically found in the ultrafine size range, this is an indication that the loss is indeed due to decreased ammonium nitrate particles. 107 The indoor exposure to ammonium nitrate particles therefore, is minimal. No conclusions could be drawn about the sulphate fragments, due to its infrequent occurrence in this study. Recommendations for Further Research For statistical accuracy, it would be favorable to obtain another year of data. Since only one set of seasonal data was obtained, there may be factors that were slightly different this year that are not representative of typical seasonal PM concentration fluctuations. Although there have been an enormous quantity of data collected, there are only four weekends to compare to weekdays at each building. Another thing that would be worth doing is sampling with a third instrument. When there are discrepancies between the PM concentration values reported by the AMS and the UHSAS, it is sometimes unclear which one is more credible. A third instrument, such as an SMPS or a CNC would not only clear up which one is giving the more accurate data, it might also give a better idea of why the two instruments do not agree at certain times. It would also give more confidence to the absolute magnitudes of ambient mass concentrations. A simple way to improve confidence in the particulate matter concentrations would be to use filter samples instead of technical equipment for comparison. If a third instrument could not be obtained, it would be worth the effort to begin each sampling campaign by measuring with both instruments some kind of control concentration, such as an ultrafine PM source that creates a consistent amount of 108 aerosols. Then if the PM concentrations seem too high or low during a certain sample week, the control can be compared to see if there is some additional factor influencing the result. This would have increased confidence in the magnitudes of outdoor and indoor particulate matter concentrations. It would be interesting to see what type of results this study would produce at better filtered buildings, such as a mechanically ventilated building with filter MERV ratings higher than 10. It would have also been beneficial to eliminate the sample locations that contained unsealed windows, as this takes away the results specific to a HVAC system infiltration. To better understand some of the ambient air quality data, it would be advantageous to perform a source apportionment on the AMS data. This method creates a best fit of possible sources to the collected data. Then certain aerosols that tend to enter the buildings can be traced to a source that could possibly be better controlled or even link health effects to indoor particulates. Another thing to do with the AMS data that is outside the scope of this project is to separate the organics into known functional groups. Without doing extra data analysis, the AMS measures organic data only as total organics. But the mass spectrum mode of the AMS obtains and stores the information necessary to separate the organics. 109 REFERENCES Chao, C. Y. H. (2001). Comparison between Indoor and Outdoor Air Contaminant Levels in Residential Buildings from Passive Sampler Study, Building And Environment. 36:999-1007. Clayton, C. A., R. L. Perritt, E. D. Pellizzari, K. W. Thomas, R. W. Whitmore, L. A. Wallace, H. Ozkaynak, and J. D. Spengler (1993). Particle Total Exposure Assessment Methodology (Pteam) Study - Distributions of Aerosol and Elemental Concentrations in Personal, Indoor, and Outdoor Air Samples in a Southern California Community, Journal Of Exposure Analysis And Environmental Epidemiology. 3:227-250. Colome, S. D., N. Y. Kado, P. Jaques, and M. Kleinman (1992). Indoor Outdoor AirPollution Relations - Particulate Matter Less Than 10 Mu in Aerodynamic Diameter (Pm-10) in Homes of Asthmatics, Atmospheric Environment Part AGeneral Topics. 26:2173-2178. Fischer, P. H., G. Hoek, H. van Reeuwijk, D. J. Briggs, E. Lebret, J. H. van Wijnen, S. Kingham, and P. E. Elliott (2000). Traffic-Related Differences in Outdoor and Indoor Concentrations of Particles and Volatile Organic Compounds in Amsterdam, Atmospheric Environment. 34:3713-3722. Harrison, R. M. and J. X. Yin (2000). Particulate Matter in the Atmosphere: Which Particle Properties Are Important for Its Effects on Health? Science Of The Total Environment. 249:85-101. Hinds, W. C., 1999: Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles. Second Edition ed. John Wiley & Sons, Inc. Holland, W. W., A. E. Bennett, I. R. Cameron, C. D. V. Florey, S. R. Leeder, R. S. F. Schilling, A. V. Swan, and R. E. Waller (1979). Special Issue on Particulate AirPollution - Health-Effects of Particulate Pollution - Reappraising the Evidence, American Journal Of Epidemiology. 110:525-659. Jayne, J. T., D. C. Leard, X. F. Zhang, P. Davidovits, K. A. Smith, C. E. Kolb, and D. R. Worsnop (2000). Development of an Aerosol Mass Spectrometer for Size and Composition Analysis of Submicron Particles, Aerosol Science And Technology. 33:49-70. Lefcoe, N. M. and Inculet, II (1975). Particulates in Domestic Premises.2. Ambient Levels and Indoor-Outdoor Relationships, Archives Of Environmental Health. 30:565-570. 110 Long, C. M. and J. A. Sarnat (2004). Indoor-Outdoor Relationships and Infiltration Behavior of Elemental Components of Outdoor Pm2.5 for Boston-Area Homes, Aerosol Science And Technology. 38:91-104. Long, C. M., H. H. Suh, P. J. Catalano, and P. Koutrakis (2001). Using Time- and Size-Resolved Particulate Data to Quantify Indoor Penetration and Deposition Behavior, Environmental Science & Technology. 35:2089-2099. Lunden, M. M., K. L. Revzan, M. L. Fischer, T. L. Thatcher, D. Littlejohn, S. V. Hering, and N. J. Brown (2003). The Transformation of Outdoor Ammonium Nitrate Aerosols in the Indoor Environment, Atmospheric Environment. 37:56335644. Morawska, L., G. Johnson, Z. D. Ristovski, and V. Agranovski (1999). Relation between Particle Mass and Number for Submicrometer Airborne Particles, Atmospheric Environment. 33:1983-1990. Mosley, R. B., D. J. Greenwell, L. E. Sparks, Z. Guo, W. G. Tucker, R. Fortmann, and C. Whitfield (2001). Penetration of Ambient Fine Particles into the Indoor Environment, Aerosol Science And Technology. 34:127-136. Phillips, J. L., R. Field, M. Goldstone, G. L. Reynolds, J. N. Lester, and R. Perry (1993). Relationships between Indoor and Outdoor Air-Quality in 4 Naturally Ventilated Offices in the United-Kingdom, Atmospheric Environment Part AGeneral Topics. 27:1743-1753. Pope, C. A. (2000). Review: Epidemiological Basis for Particulate Air Pollution Health Standards, Aerosol Science And Technology. 32:4-14. Samet, J. M., F. Dominici, F. C. Curriero, I. Coursac, and S. L. Zeger (2000). Fine Particulate Air Pollution and Mortality in 20 Us Cities, 1987-1994, New England Journal Of Medicine. 343:1742-1749. Schwartz, J. (1994). Air-Pollution and Daily Mortality - a Review and Meta Analysis, Environmental Research. 64:36-52. Seaton, A., W. Macnee, K. Donaldson, and D. Godden (1995). Particulate AirPollution and Acute Health-Effects, Lancet. 345:176-178. Spengler, J. D., D. W. Dockery, W. A. Turner, J. M. Wolfson, and B. G. Ferris (1981). Long-Term Measurements of Respirable Sulfates and Particles inside and Outside Homes, Atmospheric Environment. 15:23-30. Stein, S. W., B. J. Turpin, X. P. Cai, C. P. F. Huang, and P. H. McMurry (1994). Measurements of Relative Humidity-Dependent Bounce and Density for Atmospheric Particles Using the Dma-Impactor Technique, Atmospheric Environment. 28:1739-1746. 111 Thatcher, T. L. and D. W. Layton (1995). Deposition, Resuspension, and Penetration of Particles within a Residence, Atmospheric Environment. 29:1487-1497. Thornburg, J., D. S. Ensor, C. E. Rodes, P. A. Lawless, L. E. Sparks, and R. B. Mosley (2001). Penetration of Particles into Buildings and Associated Physical Factors. Part I: Model Development and Computer Simulations, Aerosol Science And Technology. 34:284-296. Vette, A. F., A. W. Rea, P. A. Lawless, C. E. Rodes, G. Evans, V. R. Highsmith, and L. Sheldon (2001). Characterization of Indoor-Outdoor Aerosol Concentration Relationships During the Fresno Pm Exposure Studies, Aerosol Science And Technology. 34:118-126. 112