Chapter 21 From plum puddings to Schrödinger`s cat
advertisement

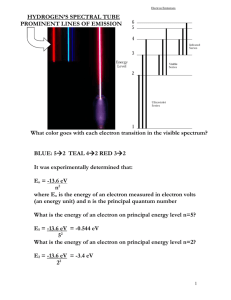
CHAPTER 21 FROM PLUM PUDDINGS TO SCHRÖDINGER'S CAT Name: QUESTIONS 21.2 The modern atom 1. Use the Balmer equation to determine the wavelength of the fourth spectral line produced in the Balmer series. 2. What will be the wavelength of the line produced by a transition from n = 5 to n = 1? 3. An ultraviolet line in the Balmer series is found to correspond to a wavelength of 3.89 × 10–7 m. What number line of the series is this? 4. Calculate the orbital radius of an electron in the fifth energy level of hydrogen. 5. What is the energy needed to remove an electron from a hydrogen atom in its sixth excited state in: (a) electron volts? (b) joules? 6. What is the energy difference between hydrogen electrons in the seventh and third excited states? 7. An electron in an excited hydrogen atom drops from the fourth to the second energy level and then from the second energy level to the ground state. Calculate the wavelength of the photons emitted by these transitions. 8. What will be the maximum wavelength of the photon that interacts with a hydrogen atom in its ground state in such a way that the atom is ionised? 9. Determine the frequency and wavelength of the photon corresponding to the fourth line of the Pfund series. 21.3 The photoelectric effect 10. Sodium has a work function equal to 2.32 eV. © John Wiley & Sons Australia, Ltd 1 QUEENSLAND PHYSICS (a) Calculate the threshold frequency of sodium. (b) If light with a wavelength of 3.6 × 10–7 m falls on sodium, what will be the kinetic energy in joules of the most energetic electrons ejected? 11. Given that the threshold frequency of zinc is equal to 9.7 × 1014 Hz, determine zinc’s: (a) threshold wavelength (b) work function. 12. When light with a wavelength of 400 nm falls on a caesium surface, the maximum velocity of the electrons ejected from the metal surface is 6.3 × 105 m s–1. What is the work function of caesium? 21.4 De Broglie’s wave theory 13. An 8.0 kg bowling ball is given a speed of 10 m s–1 as it is thrown down the bowling lane. (a) What will be its de Broglie wavelength? (b) Will it exhibit a perceptible wave nature? 14. Calculate the momentum of a particle that has a de Broglie wavelength of 5.0 × 10–12 m. 15. A particle has a momentum of 2.0 × 10–3 kg m s–1. What will be its de Broglie wavelength? Review questions Understanding 1. When even very faint light falls on the surface of a metal a photoelectron may be emitted at almost the instant that the first light strikes the surface. Explain why this cannot be satisfactorily explained by a wave model of light but can be explained if light is considered to consist of particles (photons). 2. Describe one observation that can be made in experiments investigating photoelectric effect that can be explained satisfactorily by either a wave model or a particle model of light. 3. Explain how the existence of a threshold frequency can be explained by a photon model of light. © John Wiley & Sons Australia, Ltd 2 QUEENSLAND PHYSICS 4. When de Broglie was examined for his PhD, his thesis was first thought by his examiners to bear little relationship to reality. (a) What did de Broglie predict that made it seem to be unrelated to reality? (b) What did de Broglie suggest could be observed to support his prediction? 5. If a proton and an electron are travelling with equal velocities, which has the longer de Broglie wavelength? 6. If the atoms in a sample of hydrogen were all in the state n = 5, how many different spectral lines could possibly be produced by the gas as the electrons returned to the ground state? 7. The emission spectrum of a particular gas has eight bright lines in the visible region as shown in the figure below. The absorption spectrum of the same gas has only three lines in the visible region as shown. (a) Explain why each of the absorption lines corresponds to one of the emission lines. (b) Explain why there is not a corresponding absorption line for five of the emission lines. 8. An absorption spectrum is produced when the atoms in a cool gas absorb energy from white light passing through the gas. These excited atoms then re-emit the energy and return to low energy states. How can this re-emission occur but there still be dark lines in the absorption spectrum? 9. Balmer predicted accurately the wavelengths of the visible spectral lines and invisible spectral lines of hydrogen that had not been detected. Bohr did the same about thirty years later. Why is Bohr’s prediction considered more important than that of Balmer? 10. What evidence supports the idea that the electron energies in the hydrogen atom are discrete? 11. If electrons in hydrogen atoms obeyed the rules of classical mechanics instead of those of quantum mechanics, would the hydrogen atoms produce a line spectrum or a continuous spectrum? Explain your answer. 12. Two spectral lines of hydrogen have frequencies of 2.7 × 1014 Hz (infra-red) and 4.6 × 1014 Hz (red). (a) Explain how you could use this information to determine the frequency of a higher frequency spectral line of hydrogen. © John Wiley & Sons Australia, Ltd 3 QUEENSLAND PHYSICS (b) What is the frequency of that line? 13. In what ways did the quantum mechanics developed by Heisenberg improve on the Bohr model that had successfully predicted the wavelengths of the spectral lines of hydrogen? 14. Bohr was said to be delighted after Pauli had used a new approach to calculate the wavelengths of the spectral lines of hydrogen. Suggest why Bohr might have been so pleased with Pauli’s approach. Application 15. Calculate the energy, in joules and electron volts, of photons that have the following frequencies or wavelengths: (a) f = 4.57 × 1014 Hz (b) f = 2.47 × 1015 Hz (c) λ = 632 nm (d) λ = 400 nm. 16. If the light falling on a metal surface has a frequency lower than a particular threshold frequency, no photoelectrons will be emitted, regardless of how intense the light is. Determine the value of the threshold frequency and its corresponding wavelength, and state its colour, for each of the following metals: (a) lithium, which has a work function of 2.9 eV (b) lead, which has a work function of 4.25 eV (c) tin, which has a work function of 4.42 eV (d) gold, which has a work function of 5.1 eV. 17. In an experiment to investigate the relationship between the energy of the photons incident on a clean potassium surface and the maximum energy of the emitted photoelectrons, the following results were obtained. The light was passed through 6 different filters and a retarding potential difference was applied to the photocell until the most energetic photoelectrons were stopped from passing through the cell. (a) Calculate the frequency of the photons that passed through each of the five filters that produced photoelectrons. © John Wiley & Sons Australia, Ltd 4 QUEENSLAND PHYSICS (b) Calculate the maximum energy (in joules) of the most energetic electron emitted. (c) Plot a graph of this electron energy against the frequency of the incident light. (d) From your graph: (i) determine the threshold frequency (ii) determine Planck’s constant (iii) determine the work function of potassium. 18. If one electron travels twice as fast as another electron, which one has the greater wavelength? 19. (a) (b) 20. (a) (b) If an electron travelling at 1.0 × 104 m s–1 was accelerated to 2.0 × 104 m s–1, what would be the ratio of its new wavelength to its original wavelength? If an electron travelling at 1.0 × 108 m s–1 was accelerated to 2.0 × 108 m s–1, would it change its wavelength by the same amount as the electron in part (a)? Explain your answer. Calculate the de Broglie wavelength of an electron in a TV set that hits the screen with a velocity of one tenth of the velocity of light. With what velocity would you roll a ball of mass 0.1 kg if it is to have the same de Broglie wavelength as the electron in part (a)? 21. A neutron emitted when a uranium-235 nucleus undergoes fission may have an energy of about 1 MeV. A ‘thermal’ neutron that would be captured by a uranium-235 nucleus in a nuclear reactor would have an energy of about 0.02 MeV. (a) Calculate the wavelength of a 1 MeV neutron. (b) Calculate the wavelength of a 0.02 MeV neutron. 22. Use Balmer’s equation to calculate the wavelength of the radiation emitted from an excited hydrogen atom when an electron undergoes a transition from the state n = 5 to: (a) the state n = 1 (b) the state n = 2 (c) the state n = 3. © John Wiley & Sons Australia, Ltd 5 QUEENSLAND PHYSICS 23. (a) (b) Calculate the wavelengths of the lines of the Balmer series corresponding to transitions from the states n = 8, n = 10 and n = 12. What trend do you notice in the wavelengths as the value of n increases? 24. The energies of electrons in the 5 lowest energy states of the hydrogen atom are E1 = –13.6 eV, E2 = –3.40 eV, E3 = –1.51 eV, E4 = –0.85 eV and E5 = –0.54 eV. Calculate the energies of the photons emitted and then the wavelengths of these photons for the first two lines in the: (a) Lyman series (b) Balmer series (c) Paschen series. 25. The ‘series limit’ is the term applied to the shortest wavelength spectral line in each of the spectral series of hydrogen. (Use the energies given in the previous question.) (a) What value of nI would be used to calculate the wavelength of the series limit? (b) Calculate the series limit for the Lyman, Balmer and Paschen series of hydrogen. (c) How many electron volts of energy would be carried by a photon corresponding to the series limit of the Lyman series? 26. The figure above right is an energy level diagram for energies of the stationary states in atoms of a gas Q. (a) (b) (i) Determine the energy of the photon emitted when an electron in the state n = 3 undergoes a transition to the state n = 2. (ii) Determine the frequency and wavelength of this photon. Determine the wavelength of the photon absorbed by this gas when an electron undergoes a transition from the state n = 1 to the state n = 4. Challenge © John Wiley & Sons Australia, Ltd 6 QUEENSLAND PHYSICS 27. The faintest intensity of light that can be detected by the eye is 1.5 × 10–11 watts m–2. If a light with a wavelength of 700 nm is shone into your eye at this intensity, how many photons enter your eye every second? (Assume that the average radius of the pupil is 3.5 mm.) Notes: © John Wiley & Sons Australia, Ltd 7