Chapter 18: Raman Spectroscopy
advertisement

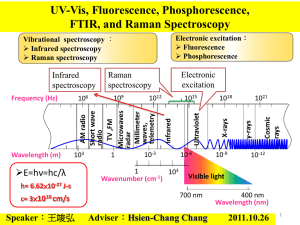
Chapter 18 Raman Spectroscopy When radiation passes through a medium, the species present scatter a fraction of the beam in every direction. In 1928, the Indian physicist, C. V. Raman discovered that the visible wavelength of a small fraction of the radiation scattered by certain molecules differs from that of the incident beam and furthermore the shifts in wavelength depend upon the chemical structure of the molecules responsible for the scattering. He was awarded the 1931 Nobel Prize in physics for this discovery. Background: The theory of Raman spectroscopy shows that the phenomenon results from the same type of quantized vibrational changes that are associated with infrared spectroscopy. Thus, the difference in wavelength between the incident and the scattered visible radiation corresponds to wavelengths in the midinfrared region. Raman and infrared absorption spectrum often resemble each other closely. There are, however, enough differences between the kinds of groups that are Raman active verses IR active. Advantages: An important advantage of Raman spectra over infrared lies in the fact that water does not cause any interference. In addition, glass or quartz cells can be employed thus avoiding working with sodium chloride or other atmospherically unstable window materials. Figure: Internal working of a spectrometer Theory of Raman Spectroscopy: Raman spectra are acquired by irradiating a sample with a powerful laser source of visible or near IR monochromatic radiation. During irradiation, the spectrum of the scattered radiation is measured at some angle (often 90 deg) with a suitable spectrometer. At the very most, the intensity of Raman lines are 0.001% intensity of the source. As a consequence, their detection and measurement are somewhat difficult than IR spectra. Mechanism of Raman and Rayleigh Scattering: In Raman spectroscopy, the spectral excitation is normally carried out by radiation having the wavelength that is well away from the absorption peaks of the analyte. Instrumentation: Instrumentation for modern Raman spectroscopy consists of three components: 1. A laser source The sources used for Raman spectroscopy are almost always lasers because their high intensity is necessary to produce Raman scattering of high enough intensity to be measured with a reasonable signal-to-noise ratio. Five of the most common laser sources for Raman spectroscopy are tabulated below: Type Source Wavelength, nm Argon ion 488.0 or 514.5 Krypton ion 530.0 or 647.1 Helium/Neon 635.8 Diode Laser 782 or 830 Nd/YAG 1064 2. A sample illumination system Sample handling for Raman spectroscopic measurements is simpler than for IR spectroscopy because glass can be used for windows, lens and other optical components, rather than the more fragile atmospherically unstable crystalline halides. 3. A suitable spectrometer Both the Fourier Transform instruments and the Dispersion instruments are good for measurement on samples. References: American Chemical Society: http://www.acs.org Chemical Abstracts Service: http://www.cas.org Chemical Center Home Page: http://www.chemcenter/org Science Magazine: http://www.sciencemag.org Journal of Chemistry and Spectroscopy: http://www.kerouac.pharm.uky.edu/asrg/wave/wavehp.html