Bonus Problem 4
advertisement
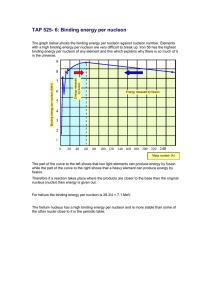
Paul Avery PHY3101 Fall 2015 Name (PRINT): Bonus Problem 4 Due Monday, Oct. 12, 2015 For all problems (1) Put your name clearly in the printout or file (2) Describe what software you used (3) Show the program, including a screenshot or printout. Make sure it is readable with large fonts and good contrast. I can’t grade it if I can’t easily read the screenshot. 1. (10 pts) Consider the potential function Φ ( x, w) = 1 1− 2xw + w2 This can be expanded as a power series in w with coefficients that depend on x. Φ ( x, w) = 1 1− 2xw + w2 = ∑ n An ( x ) w Find the functions An ( x ) for n = 0 through 10 in factored form. 1 n 2. The semiempirical mass formula is an estimate of the binding energy B of a nucleus given its atomic number Z and mass number A = Z + N, where N is the number of neutrons. It is most accurate for heavier nuclei than for light nuclei. From the binding energy and mass of the constituents, we can calculate the actual nuclear mass: M = Zm p + ( A − Z ) mn − B / c 2 . The semiempirical formula is approximately 2/3 B = aV A − aS A ⎧ +δ 0 ⎪⎪ δ ( A,Z ) = ⎨ 0 ⎪ −δ 0 ⎪⎩ − aC Z 2 A1/3 − a A ( A − 2Z ) A 2 + δ ( A,Z ) A even, Z even A odd A even, Z odd where all coefficients are in units of MeV. One fit to the datai gives aV = 15.75 , aS = 17.8 . aC = 0.711 , a A = 23.7 and δ 0 = 11.8 . a. (5 pts) For each element from Z = 1 to Z = 100, calculate the binding energy with the number of neutrons going from 1 to 2Z and save in a two dimensional array. Find for each element the state with the highest binding energy/nucleon (this is the most stable). Print out for each element this value of (Z,A), binding energy, binding energy/nucleon. b. (5 pts) For each element, print out (Z,A), binding energy, binding energy/nucleon for the 5 most stable isotopes (using your values calculated here). Print in a way that’s easy to read. c. (5 pts) For each A value, print out (Z,A), binding energy, binding energy/nucleon for the 5 most stable isobars (using your values calculated here). Print in a way that’s easy to read. i See en.wikipedia.org/wiki/Semi-empirical_mass_formula for an explanation of these terms, which we will consider later in the course 2