obtained electrons
advertisement

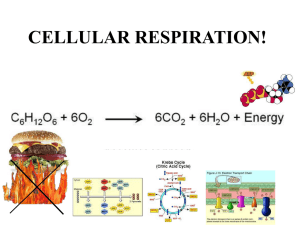
Bio 225 Chapter 5 Practice Questions 1. Chemical reactions that break down complex molecules into simpler molecules and usually release energy are: A. Dehydration reactions B. Reduction reactions C. Anabolic reactions D. Metabolic reactions E. Catabolic reactions 2. What kind of chemical reactions result in the synthesis of a protein from amino acids? A. Hydrolysis reactions B. Oxidation reactions C. Anabolic reactions D. Metabolic reactions E. Catabolic reactions 3. Which of the following statements is false? A. Anabolism + catabolism = metabolism B. ATP → ADP + P + energy is a catabolic reaction C. ADP + P + energy → ATP is an anabolic reaction D. 3 Fatty acids + 1glycerol + ATP → 1Triglyceride + 3 H2O + ADP + P is an anabolic reaction E. All are true Look at the following diagram: E1 A → E2 B → E3 C → D The arrows represent chemical reactions, the letters A-D represent substrates and products, and E1, E2, and E3 represent the enzymes that catalyze the chemical reactions. 4. Which of the following statements is not supported (could be true but also might not be true, just don’t have enough information to tell) by the information given? A. The entire diagram represents a metabolic pathway B. A is a substrate, B is a product C. B is the substrate for the second chemical reaction D. An organism that has this pathway present will have genes that code for E1, E2, and E3 E. This is an anabolic pathway 5. What is an enzyme? A. A biological catalyst B. The same thing as a substrate C. An intermediate in a catabolic pathway D. A molecule that speeds up chemical reactions E. Both A and D are correct 6. Enzymes are usually: A. Proteins B. Lipids C. Carbohydrates D. Nucleic acids E. Either A or D 7. Which of the following statements regarding enzymes is false? A. Enzymes are not used up in a chemical reaction B. Enzymes decrease the activation energy required for a reaction C. Enzymes increase the probability of a reaction D. Enzymes function as electron acceptors in oxidation-reduction reactions E. All of the statements are true, none are false 8. A coenzyme is: A. The second enzyme in a reaction that requires two enzymes B. One of several enzymes in a multi-step metabolic pathway C. The non-protein part of a holoenzyme D. The same thing as an apoenzyme 9. The specificity of an enzyme is determined by: A. The shape of the active site B. The coenzyme C. The product D. The allosteric site E. The substrate 10. The allosteric site is where: A. The substrate binds B. The product is released C. A competitive inhibitor binds D. The coenzyme binds E. A noncompetitive inhibitor binds 11. Feedback inhibition occurs when: A. The substrate blocks the active site B. The feedback inhibitor binds to the active site C. The product binds to the allosteric site D. The feedback inhibitor binds to the coenzyme 12. Which of the following is least likely to decrease enzyme activity? A. Too much heat B. Too much acid C. Too much base D. Too much salt E. Too much substrate 13. Ribozymes are: A. The part of a holoenzyme that contains the allosteric site B. The part of the apoenzyme that contains the active site C. Enzymes that synthesize ribosomes D. Enzymes that synthesize proteins on ribosomes E. None of the above 14. In aerobic cellular respiration the final electron acceptor is: A. H2O B. ATP C. CO2 D. NAD+ E. O2 15. In anaerobic cellular respiration the final electron acceptor is: A. CO2 B. O2 C. An organic molecule D. An inorganic molecule other than O2 16. In fermentation the final electron acceptor is: A. CO2 B. O2 C. An organic molecule D. An inorganic molecule other than O2 17. In cellular respiration chemiosmosis is powered by a proton gradient that is set up by: A. Substrate level phosphorylation B. Lactic acid synthesis C. CO2 fixation D. Reduction of oxidized chlorophyll E. High energy electrons obtained from the oxidation of organic molecules 18. Substrate level phosphorylation is: A. The indirect transfer of high energy phosphate molecules down a chain of carriers and eventually on to ADP B. Direct transfer of phosphate from a substrate to ADP C. Direct transfer of phosphate from ATP to a substrate D. The same as chemiosmosis 19. The process that involves transfer of electrons from organic compounds down an electron transport chain to a final electron acceptor and harnessing the energy released during electron transfer to make ATP by chemiosmosis is: A. Substrate level phosphorylation B. Photophosphorylation C. Oxidative phosphorylation 20. In oxidative phosphorylation high energy electrons are obtained by: A. Oxidation of organic compounds B. Oxidation of chlorophyll C. Excitement by light D. Oxidation of ATP E. Chemiosmosis 21. In photophosphorylation high energy electrons are obtained by: A. Oxidation of organic compounds B. Oxidation of chlorophyll C. Excitement by light D. Oxidation of ATP E. Chemiosmosis 22. In fermentation high energy electrons are obtained by: A. Oxidation of organic compounds B. Oxidation of chlorophyll C. Excitement by light D. Oxidation of ATP E. Chemiosmosis 23. In fermentation high energy electrons are used to make ATP. A. True B. False 24. In fermentation, ATP is generated by: A. Oxidative phosphorylation B. Photophosphorylation C. Substrate level phosphorylation D. Magic E. Wishful thinking 25. Glucose is not used to produce ATP in: A. Glycolysis B. The Enter-Douderoff pathway C. The Calvin-Benson cycle D. Beta-oxidation E. C and D 26. Which of the following is not produced by glycolysis? A. Two net ATP molecules B. Two NADH molecules C. Two pyruvate molecules D. Two acetyl-CoA molecules E. All are products of glycolysis 27. In aerobic cellular respiration, pyruvic acid is converted to: A. Sodium chloride and water B. Lactic acid C. Acetyl-CoA D. NADH E. ATP 28. In fermentation, pyruvic acid may be converted to: A. Sodium chloride and water B. Lactic acid C. Acetyl-CoA D. NADH E. ATP 29. One ATP, three NADH, and one FADH2 are produced by: A. The Krebs cycle B. Glycolysis C. The electron transport chain D. The Calvin-Benson cycle E. The Entner-Douderoff pathway 30. The starting molecule that enters the Krebs cycle is: A. Acetyl-CoA B. Oxygen C. Glucose D. Pyruvic acid E. Lactic acid in the absence of oxygen 31. In cellular respiration and photophosphorylation, the proton pumps that move hydrogen ions across a membrane to establish a proton gradient are energized by: A. Chemiosmosis B. The Calvin-Benson cycle C. Chlorophyll D. NADPH E. An electron transport chain 32. Chemiosmosis: A. Uses ATP to pump hydrogen ions across a membrane B. Is the source of most ATP generated by substrate level phosphorylation C. Is made possible by the passage of protons down the electron transport chain D. None of the above 33. Chemiosmosis requires: A. Unequal hydrogen ion distribution across the membrane B. Oxygen C. ATP D. NADPH 34. NADH is reduced, NAD+ is oxidized. A. True B. False 35. NAD+ is required so that: A. Substrate level phosphorylation can occur B. Pyruvate can be converted to acetyl-CoA C. The Krebs cycle can function D. Fermentation can occur E. All of the above F. A, B, and C only G. B, C, and D only H. C, and D only 36. The number of ATP produced from one molecule of glucose by substrate level phosphorylation in glycolysis is: A. 0 B. 1 C. 2 D. 3 E. 34 F. 36 G. 38 37. The number of pyruvic acid molecules produced from one glucose molecule in glycolysis is: A. 0 B. 1 C. 2 D. 3 E. 34 F. 36 G. 38 38. The number of acetyl-CoA molecules produced from one pyruvic acid molecule during aerobic cellular respiration is: A. 0 B. 1 C. 2 D. 3 E. 34 F. 36 G. 38 39. The number of ATP produced from one molecule of acetyl-CoA by substrate level phosphorylation in the Krebs cycle is: A. 0 B. 1 C. 2 D. 3 E. 34 F. 36 G. 38 40. The number of ATP produced from one molecule of glucose by substrate level phosphorylation in the Krebs cycle is: A. 0 B. 1 C. 2 D. 3 E. 34 F. 36 G. 38 41. The number of ATP produced from one molecule of glucose by substrate level phosphorylation in aerobic cellular respiration in prokaryotes is: A. 0 B. 2 C. 3 D. 4 E. 34 F. 36 G. 38 42. The number of ATP produced from one molecule of glucose by substrate level phosphorylation in fermentation is: A. 0 B. 1 C. 2 D. 3 E. 34 F. 36 G. 38 43. The number of ATP produced from one molecule of glucose by oxidative phosphorylation in fermentation is: A. 0 B. 1 C. 2 D. 3 E. 34 F. 36 G. 38 44. The number of ATP produced by oxidative phosphorylation when one NADH donates a pair of electrons to the electron transport chain and chemiosmosis occcurs is: A. 0 B. 1 C. 2 D. 3 E. 34 F. 36 G. 38 45. The number of ATP produced by oxidative phosphorylation when one FADH 2 donates a pair of electrons to the electron transport chain and chemiosmosis occurs is: A. 0 B. 1 C. 2 D. 3 E. 34 F. 36 G. 38 46. The number of ATP produced from one molecule of glucose by oxidative phosphorylation in aerobic cellular respiration in prokaryotes is: A. 0 B. 1 C. 2 D. 3 E. 34 F. 36 G. 38 47. Organisms that use fermentation to make ATP and organisms that use cellular respiration to make ATP are least likely to have which of the following in common? A. A final electron acceptor B. Substrate level phosphorylation C. An electron transport chain and chemiosmosis D. Glycolysis E. Oxidation of organic molecules to release high energy electrons 48. Beta-oxidation: A. Produces acetyl-CoA for gluconeogenesis B. Metabolizes the glycerol portion of triglycerides C. Splits triglycerides into fatty acids and glycerol D. Links acetyl-CoA molecules together to generate fatty acids E. Oxidizes fatty acids to acetyl-CoA molecules 49. Which of the following statements regarding protein catabolism are true? A. All amino acids must be deaminated to enter catabolic pathways B. Some amino acids may be converted to acetyl-CoA C. Some amino acids may be converted to intermediates in glycolysis D. Some amino acids may be converted to intermediates in the Krebs cycle E. All of the above F. None of the above 50. The whole point of photosynthesis is to produce (as an end product): A. ATP B. Ribulose diphosphate C. Oxygen D. CO2 E. Glucose 51. Cyclic photosynthetic reactions produce ATP and NADH while noncyclic photosynthetic reactions produce ATP and NADPH. A. True B. False 52. The Calvin-Benson cycle produces: A. ATP B. NADPH C. CO2 D. O2 E. Glucose 53. CO2 fixation occurs in: A. Cyclic photosynthetic reactions B. Noncyclic photosynthetic reactions C. Anoxygenic photosynthetic reactions D. The Calvin-Benson cycle 54. Oxygen is produced from the oxidation of H2O in: A. Certain cyclic photosynthetic reactions B. Certain noncyclic photosynthetic reactions C. Anoxygenic photosynthetic reactions D. The Calvin-Benson cycle 55. Organisms that use photophosphorylation to make ATP and organisms that use cellular respiration to make ATP are least likely to have which of the following in common? A. Oxygen as the final electron acceptor B. An electron transport chain C. Chemiosmosis D. Glycolysis 56. Organisms that use organic compounds as a carbon source are: A. Phototrophs B. Chemotrophs C. Autotrophs D. Heterotrophs 57. Organisms that use light as an energy source are: A. Phototrophs B. Chemotrophs C. Autotrophs D. Heterotrophs 58. Organisms that use organic compounds as an energy source are: A. Phototrophs B. Chemotrophs C. Autotrophs D. Heterotrophs 59. Organisms that use CO2 as a carbon source are: A. Phototrophs B. Chemotrophs C. Autotrophs D. Heterotrophs 60. Glucose may be synthesized from intermediates in glycolysis and the Krebs cycle. A. True B. False 61. Glycogen is synthesized from: A. UDPG B. UDPNAc C. Glycerol + fatty acids D. Amino acids 62. Peptidoglycan is synthesized from: A. UDPG B. UDPNAc C. Glycerol + fatty acids D. ADPG 63. Triglycerides are synthesized from: A. UDPG B. UDPNAc C. Glycerol + fatty acids D. ADPG 64. Amino acids may be synthesized from: A. Intermediates in glycolysis B. Pentose phosphate pathway intermediates C. Kreb cycle intermediates D. Entner-Douderoff pathway intermediates E. All of the above 65. Purine and pyrimidine nucleotides are synthesized from sugars derived from the pentose phosphate pathway or the Entner-Douderoff pathway and: A. Glycogen B. Glycerol C. Fatty acids D. Peptidoglycan E. Certain amino acids Bio 225 Chapter 5 Practice Exam Key 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 e 34 a c 35 g e 36 c e; You can't tell what kind of 37 c pathway it is from the 38 b information given. 39 b e 40 c; Not the glucose itself, the 2 AcCoA’s you get from the glucose a; we’ll do ribozymes in the next unit. 41 d d 42 c c 43 a a 44 d e 45 c c 46 e e 47 c e 48 e e 49 e d 50 e c 51 b e 52 e b 53 d c 54 b a 55 d c 56 d a 57 a b 58 b c 59 c e 60 a d 61 a c 62 b b 63 c a 64 e a 65 e e d d; gotta have a proton gradient to generate a proton motive force