Newby From Patient to Payment 5th Edition Chapter 8
advertisement

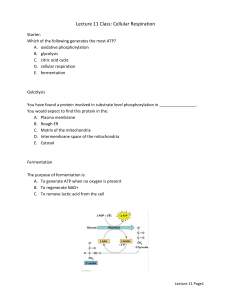
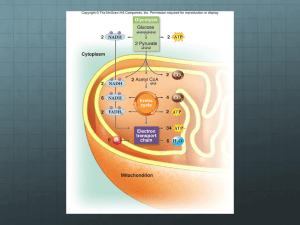
Biology Brooker/Widmaier/Graham/Stiling 1st Edition Chapter 7 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Question 1 The sum total of all reactions that occur within a cell is referred to as: A) the Central Dogma. B) metabolism. C) respiration. D) thermodynamics. E) oxidative phosphorylation. Question 2 The Blue Cross and Blue Shield medical insurance policy is called a certificate. A. True B. False Question 3 The synthesis of ATP using energy provided by an electrochemical gradient is most accurately referred to as: A) substrate level phosphorylation. B) reduction. C) oxidation. D) oxidative phosphorylation. E) fermentation. Question 4 Which one of the following does not represent a likely function of a secondary metabolite? A) producing a bad taste to prevent an animal from eating a plant B) attracting insects to assist in pollination C) aiding in the formation of a cell wall D) inhibiting growth of nearby organisms E) All of the above are likely roles of secondary metabolites. Question 5 Match the type of energy on the left with the appropriate description on the right. i. potential energy a. energy contained within covalent bonds ii. chemical energy b. energy associated with movement c. energy due to structure or location iii. kinetic energy A) i = c; ii = b; iii = a B) i = a; ii = c; iii = b C) i = a; ii = b; iii = c D) i = b; ii = a; iii = c E) i = c; ii = a; iii = b Question 6 Which one of the following describes a reaction in which the products are at a higher energy level than the reactants? A) endergonic B) -DG C) spontaneous D) catabolic E) hydrolysis of ATP Question 7 True or False: While the majority of biological catalysts are proteins, some catalytic activity can be found in nucleic acid molecules. A) True B) False Question 8 Which one of the following is statements about enzymes is incorrect? A) Enzymes can temporarily accept reactive groups from substrate molecules. B) Enzymes are optimally active within a narrow range of pH and temperature. C) Substrates bind very specifically to an enzyme's active site. D) Enzymes allow reactions to occur that would not happen in the absence of enzyme. E) The binding of a substrate molecule to an enzyme often puts a strain on the bonds within the substrate. Question 9 The gain of electrons is referred to as ________, while the loss of electrons is referred to as ________. A) oxidation; reduction B) condensation; hydrolysis C) reduction; oxidation D) dephosphorylation; phosphorylation E) hydrolysis; condensation Question 10 Which one of the following statements about ATP is correct? A) Approximately 80% of all cellular proteins are believed to bind ATP. B) A given molecule of ATP in an average human is believed to undergo 10,000 rounds of hydrolysis and re-synthesis per day. C) Chaperones are regulatory proteins that use ATP to attach a phosphate to proteins. D) The synthesis of ATP from ADP and inorganic phosphate is a spontaneous reaction. E) None of the above statements about ATP is correct. Question 11 True or False: The product of a metabolic pathway inhibiting an enzyme that acts earlier in the pathway is an example of competitive inhibition. A) True B) False Question 12 The greatest impact on the formation of the product of a metabolic pathway is generally seen at the: A) first step. B) last step. C) rate-limiting step. D) most exergonic step. E) most endergonic step. Question 13 Which one of the following statements about glycolysis is incorrect? A) Glycolysis involves an energy "investment" phase and an energy "payout" phase. B) A net yield of 2 ATP is achieved per molecule of glucose in glycolysis. C) At the end of glycolysis, the six carbons that originated in glucose are found in one molecule of pyruvate and three carbon dioxides. D) Glycolysis is essentially the same in all living organisms. E) Some of the energy liberated during glycolysis is used to reduce NAD+ into NADH. Question 14 In eukaryotes, pyruvate oxidation occurs in the _______, and the citric acid cycle occurs in the _______. A) cytoplasm; mitochondrial matrix B) mitochondrial matrix; mitochondrial matrix C) outer mitochondrial membrane; inner mitochondrial membrane D) cytoplasm; cytoplasm E) outer mitochondrial membrane; mitochondrial matrix Question 15 True or False: The final electron acceptor at the end of the electron transport chain is oxygen. A) True B) False Question 16 Which one of the following is not a product of the citric acid cycle? A) FADH2 B) NADH C) carbon dioxide D) GTP (then converted to ATP) E) water Question 17 Which one of the following components of the electron transport chain does not actively pump protons across the inner mitochondrial membrane? A) cytochrome b-c1 B) cytochrome oxidase C) NADH dehydrogenase D) succinate reductase E) All of the above components of the electron transport chain actively pump protons across the inner mitochondrial membrane. Question 18 True or False: Energy can be obtained from food products other than carbohydrates by converting those food sources into molecules that enter glycolysis or the citric acid cycle at other points. A) True B) False Question 19 Which one of the following statements about anaerobic respiration is correct? A) Cells undergoing anaerobic respiration generate additional ATP during fermentation reactions. B) Yeast cells build up lactic acid as a by-product of anaerobic respiration. C) An important function of fermentation reactions is to recycle FADH2 back to FAD. D) Some organisms have evolved methods of using molecules other than oxygen as terminal electron acceptors. E) Since there is no oxygen in the ocean, all marine life must be capable of carrying out anaerobic respiration. Question 20 Match the type of secondary metabolite on the left with the representative examples on the right. a. beta-carotene, cinnamon i. polyketides b. capsaicin, atropine ii. alkaloids c. streptomycin, tetracycline iii. phenolic compounds d. vanillin, pelargonidin iv. terpenoids A) i = c; ii = b; iii = d; iv = a B) i = b; ii = a; iii = c; iv = d C) i = d; ii = c; iii = a; iv = b D) i = a; ii = b; iii = d; iv = c E) i = d; ii = b; iii = c; iv = a Answer Key 1. B 2. E 3. D 4. C 5. E 6. A 7. A 8. D 9. C 10. B 11. B 12. C 13. C 14. B 15. A 16. E 17. D 18. A 19. D 20. A