Bright Line Spectrum Lab Handout
advertisement

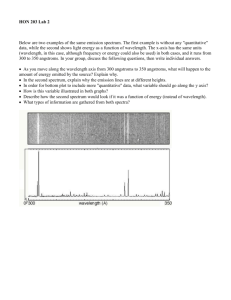
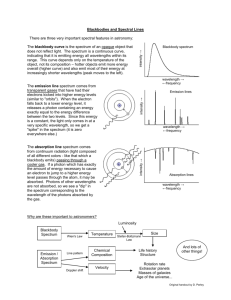
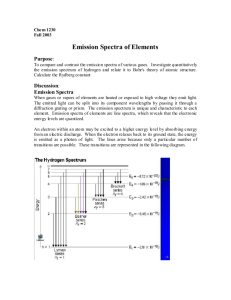
Bright Line Spectrum Lab Name______________________ Background: About 100 years ago, a Danish physicist by the name of Niels Bohr, performed experiments on hydrogen atoms and measured the wavelengths of the emission spectra. There are three types of spectra: 1. Continuous Spectrum- produced by a glowing solid, liquid, or gas under certain conditions. This spectrum consists of a continuous set of emission lines side by side, with no gaps, and appearing as a smooth transition of all colors from red to violet. 2. Dark-Line Spectrum / Absorption Spectrum- produced when a cooler gas lies between the observer and an object emitting a continuous spectrum. The cooler gas absorbs specific wavelengths of radiation passing through it. This spectrum appears as a continuous spectrum of all colors with a number of gaps or dark lines in it. We will not be using this type in this lab. 3. Bright-Line Spectrum / Emission Spectrum- produced by a glowing gas which radiates energy at specific wavelengths characteristic of the element or elements composing the gas. This spectrum consists of a number of bright lines against a dark background. Different elements produce different spectra. These different spectra are called the atomic spectra and are unique enough to be thought of as a finger print of the element. In this experiment, you will be making measurements of the wavelengths of the third type of spectra and use those measured wavelengths to calculate the energy associated with the transition of an electron in the hydrogen atom from one energy level to another. Purpose: The purpose of this lab is two-fold. One is to observe spectra of known elements, record wavelengths of spectra observed and to identify unknowns using the data obtained. The second part is to calculate the energy associated with each line observed in the hydrogen spectrum and then calculate the Rydberg constant associated with that particular wavelength of light. Materials: Various gas discharge tubes, power supply, spectroscopes. Procedure: 1. 2. Draw the bright line spectra for each element observed. Pay particular attention to the wavelengths of light that you see. Using your data, identify several unknowns. Pre-lab Questions: 1. The visible region of the electromagnetic spectrum lies between the wavelengths of ______ and ______ nm. 2. A certain photon has a wavelength of 550 nm. Calculate its energy in Joules. 3. Calculate the energy (J) and wavelength (nm) of the photon emitted by the 4→1 transition in a hydrogen atom. Are you able to see this photon of light? Justify your answer. Data: Hydrogen: 700 | 650 | Wavelength in nm 625 600 575 | | | 650 | 625 | 650 | Wavelength in nm 625 600 575 | | | 675 | 650 | 675 | 675 | 550 | 525 | 500 | 475 | 450 | 425 | 400 | Wavelength in nm 600 575 550 | | | 525 | 500 | 475 | 450 | 425 | 400 | 550 | 525 | 500 | 475 | 450 | 425 | 400 | Wavelength in nm 625 600 575 | | | 550 | 525 | 500 | 475 | 450 | 425 | 400 | 650 | Wavelength in nm 625 600 575 | | | 550 | 525 | 500 | 475 | 450 | 425 | 400 | 650 | Wavelength in nm 625 600 575 | | | 550 | 525 | 500 | 475 | 450 | 425 | 400 | Helium: 700 | 675 | Oxygen: 700 | 675 | Nitrogen: 700 | Neon: 700 | Mercury: 700 | 675 | Unknown: Sample # 1 700 | 675 | 650 | Wavelength in nm 625 600 575 | | | Unknown: Sample # 2 700 | 675 | 650 | 675 | 650 | 550 | 525 | 500 | 475 | 450 | 425 | 400 | Identity of element:______________________________ Wavelength in nm 625 600 575 | | | Unknown: Sample # 3 700 | Identity of element:______________________________ 550 | 525 | 500 | 475 | 450 | 425 | 400 | Identity of element:______________________________ Wavelength in nm 625 600 575 | | | 550 | 525 | 500 | 475 | 450 | 425 | 400 | Analysis: 1. Use the energy diagram below to answer the following questions: 5 4 3 2 1 a) Draw arrows on the diagram to show all of the energy level transitions an electron could make that would result in the emission of a photon. b) How many emission lines could be observed from this atom? c) Which transition would emit light with the highest frequency (shortest wavelength)? From n = _____ to n = ______ d) Which transition would emit light with the lowest frequency (longest wavelength)? From n = _____ to n = ______ 2. Calculate the energy associated with each bright line observed in the hydrogen atom. 3. Calculate the Rydberg constant for each line observed in the hydrogen atom and calculate an average. Look up the accepted value of the Rydberg constant and calculate a percent error from your observations. 4. Explain why the elements give a bright line spectrum rather than a continuous spectrum. 5. Why does electricity have to be applied to the gas tube before the element emits light?