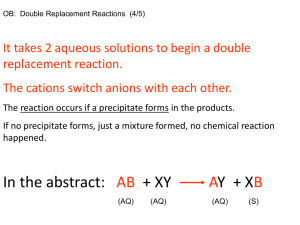
Chemical Equations and Solubility
advertisement

Chem 1 Chemical Equations Practice Quiz 1. A solid produced by a chemical reaction in solution that separates from the solution is called a) a precipitate b) a reactant c) a molecule d) the mass of the product 2. In writing an equation that produces hydrogen gas, the correct representation of hydrogen gas is a) H b) 2H c) H2 d) OH 3. To balance a chemical equation, it may be necessary to adjust the a) coefficients b) subscripts c) formulas of the products d) number of products 4. A chemical equation is balanced when the a) coefficients of the reactants equal the coefficients of the products b) same number of each kind of atom appears in the reactants and in the products c) products and reactants are the same chemicals d) subscripts of the reactants equal the subscripts of the products 5. How would oxygen be represented in the chemical equation for the reaction of methane and oxygen gas to yield carbon dioxide and water? a) oxygen b) O c) O2 d) O3 e) 2O 6. Which of the following is the correct chemical equation for the formation of carbon dioxide from carbon and oxygen? a) 2C + 2O2 --> CO2 b) C + O2 ---> CO2 c) CO2 ---> C + O2 d) 2C + O ---> CO2 7. In an equation, the symbol for a substance in a water solution is followed by a) (l). b) (g). c) (aq). d) (s). 8. When the equation Fe3O4 + Al --> Al2O3 + Fe is correctly balanced, what is the coefficient of Fe? a) 3 b) 4 c) 6 d) 9 9. Which coefficients correctly balance the formula equation NH4NO2 --> N2 (g) + H2O ? a) 1,2,2 b) 1,1,2 c) 2,1,1 d) 2,2,2 10. Which coefficients correctly balance the formula equation CaO + H2O ---> Ca(OH) 2? a) 2,1,2 b) 1,2,3 c) 1,2,1 d) 1,1,1 11. The complete balanced equation for the reaction between zinc II hydroxide and acetic acid is a) ZnOH + CH3COOH ---> ZnCH3COO + H2O b) Zn(OH)2 + CH3COOH ---> Zn + 2CO2 + 3H2O c) Zn(OH)2 + 2CH3COOH ---> Zn(CH3COO)2 + 2H2O d) Zn(OH)2 + 2CH3COOH ---> Zn(CH3COO)2 + O2 + H2 12. Which equation is NOT balanced? a) 2H2 + O2 ---> 2H2O b) 4H2 + 2O2 ---> 4H2O c) 2Al + 3F2 -->2AlF3 d) 2H2 + O2 ---> H2O 13. In what kind of reaction do two elements combine to form a new compound? a) decomposition reaction b) ionic reaction c) double-replacement reaction d) synthesis reaction 14. The equation AX ---> A + X is the general equation for a a) synthesis reaction b) decomposition reaction c) combustion reaction d) single-replacement reaction 15. In what kind of reaction does a pure element replace a similar element in a compound? a) single-replacement reaction b) double-replacement c) decomposition reaction d) ionic reaction 16. The equation AX + BY ---> AY + BX is the general equation for a(n) a) synthesis reaction b) decomposition reaction c) single-replacement reaction d) double-replacement reaction 17. 2Al (s) + 3H2 (g) --> 2AlH3 (s) is what type of reaction? a) combustion reaction b) ionic reaction c) synthesis reaction d) double-replacement reaction 18. In what kind of reaction does a single compound produce two or more simpler substances? a) decomposition reaction b) synthesis reaction c) single-replacement reaction d) ionic reaction 19. F2 + 2NaI --> 2NaF + I2 is what type of reaction? a) double-replacement reaction b) decomposition reaction c) single-replacement reaction d) ionic reaction 20. In what kind of reaction do the ions of two compounds exchange places in aqueous solution to form two new compounds? a) synthesis reaction b) double-replacement reaction c) decomposition reaction d) combustion reaction 21. The reaction 2Mg(s) + O2 (g) ----> 2MgO(s) is a a) synthesis reaction b) decomposition reaction c) single-replacement reaction d) double-replacement reaction 22. The reaction Mg (s) + 2HCl (aq) --> H2 (g) + MgCl2 (aq) is a a) composition reaction b) decomposition reaction c) single-replacement reaction d) double-replacement reaction 23. The reaction 2HgO(s)---> 2Hg(l) + O2 (g) is a(n) a) single-replacement reaction. b) synthesis reaction. c) ionic reaction. d) decomposition reaction. 24. The reaction Pb(NO3) 2 (aq) + 2KI(aq)----> PbI2 (s) + 2KNO3 (aq) is a a) double-replacement reaction. b) synthesis reaction. c) decomposition reaction. d) combustion reaction. 25. The reaction 2KClO3 (s) ---> 2KCl(s) + 3O2 (g) is a(n) a) synthesis reaction. b) decomposition reaction. c) combustion reaction. d) ionic reaction. 26. The reaction Cl2 (g) + 2KBr(aq) ---> 2KCl(aq) + Br2 (l) is a(n) a) synthesis reaction. b) ionic reaction. c) single-replacement reaction. d) combustion reaction. 27. Iron II sulfide reacts with hydrochloric acid to form Iron II chloride and hydrogen sulfide. Which of the following is the correct chemical equation that shows this (don’t worry about balancing)? a) Fe2S + HCl FeCl2 + H2S b) FeS2 + HCl FeCl2 + H2S c) FeS + HCl FeCl + H2S d) FeS + HCl FeCl2 + H2S e) FeS2 + H2SO4 FeCl2 + H2S 28. When a binary compound decomposes, what is produced? a) an oxide b) an acid c) a tertiary compound d) two elements 29. In the equation 2Al(s) + 3Fe(NO3)2 (aq) ---> 3Fe(s) + 2Al(NO3)3 (aq), iron has been replaced by a) nitrate. b) water. c) aluminum. d) nitrogen. 30. When an insoluble solid compound is produced in a double-replacement reaction, a a) gas bubbles off. b) precipitate is formed. c) combustion reaction takes place. d) halogen is produced. 31. In a double-replacement reaction, hydrogen chloride and sodium hydroxide react to produce sodium chloride. The other product is a) sodium hydride. b) potassium chloride. c) water. d) hydrogen gas. 32. When potassium reacts with water, one product formed is a) hydrogen gas. b) oxygen gas. c) potassium oxide. d) salt. 33. A precipitate forms in a double-replacement reaction when a) hydrogen gas reacts with a metal. b) positive ions combine with negative ions. c) water boils out of the solution. d) a gas escapes. 34. What is the balanced equation when aluminum reacts with copper(II) sulfate? a) Al + Cu2S ---> Al2S + Cu b) 2Al +3CuSO4 ---> Al2 (SO4)3 + 3Cu c) Al + CuSO4 ---> AlSO4 + Cu d) 2Al + Cu2SO4 ---> Al2SO4 + 2Cu 35. Which compound dissociates to produce the ions SO42- (aq) and NH4+ (aq)? a) NH4SO4 (s) b) (NH4)2SO4 (s) c) NH4 (SO4)2 (s) d) (NH4)3 (SO4)2 (s) 36. Which compound dissociates to produce the ions Ca2+ (aq) and NO3- (aq)? a) CaNO3 (s) b) Ca2NO3 c) Ca(NO3)2 (s) d) Ca2(NO3)3 37. Which of the following compounds is NOT soluble in water? a) FeCl3 b) Cu(OH)2 c) NaI d) Pb(NO3)2 e) KC2H3O2 38. Which of the following compounds will dissolve in water? a) K3PO4 b) Mg3(PO4)2 c) CuS d) ZnCO3 e) PbBr2 39. Which of the following pairs of solutions produce a precipitate when combined? a) Cu(NO3)2 and NaCl b) Fe(NO3)3 and MgCl2 c) Cu(NO3)2 and K2CO3 d) CaCl2 and NaNO3 40. Which of the following pairs of solutions produce a precipitate when combined? a) KOH and NH4Cl b) Fe(NO)3 and KCl c) Na2SO4 and KCl d) NH4Cl and AgNO3 41. When solutions of Pb(NO3)2 and Fe2(SO4)3 are combined, what precipitate(s) forms? a) PbSO4 b) Fe(NO3)3 c) FeNO3 d) Pb2(SO4)3 e) none of the above 42. When solutions of NH4OH and K2SO4 are combined, what precipitate(s) forms? a) (NH4)2SO4 b) KOH c) K2OH d) K(NH4)2 e) none of the above 43. When the hydrocarbon C8H18 is burned in oxygen gas, what is the coefficient for oxygen in the balanced equation? a) 3 b) 6 c) 12 d) 18 e) 25 44. When Aluminum metal is placed in a solution of Nickel II Sulfate, pure nickel is produced along with what other compound? a) Al2O3 b) Al2(SO4)3 c) NiSO4 d) AlSO4 e) Al3(SO4)2 45. Which of the following is sodium phosphate? a) NaP b) Na3P c) NaPO4 d) Na3PO4 e) Na(PO4)3 ----------Key---------1. (a) 2. (c) 3. (a) 4. (b) 5. (c) 6. (b) 7. (c) 8. (d) 9. (b) 10. (d) 11. (c) 12. (d) 13. (d) 14. (b) 15. (a) 16. (d) 17. (c) 18. (a) 19. (c) 20. (b) 21. (a) 22. (c) 23. (d) 24. (a) 25. (b) 26. (c) 27. (d) 28. (d) 29. (c) 30. (b) 31. (c) 32. (a) 33. (b) 34. (b) 35. (b) 36. (c) 37. (b) 38. (d) 39. (c) 40. (d) 41. (a) 42. (e) 43. (e) 44. (b) 45. (d)