KMT Questions: Particle Theory Worksheet
advertisement
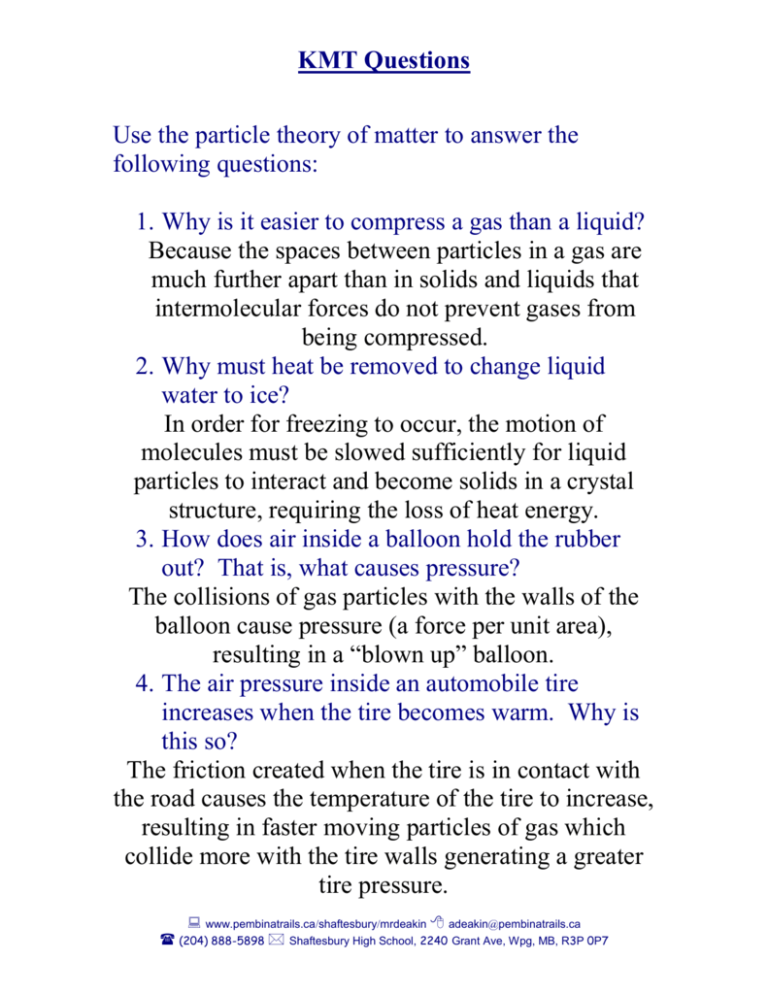
KMT Questions Use the particle theory of matter to answer the following questions: 1. Why is it easier to compress a gas than a liquid? Because the spaces between particles in a gas are much further apart than in solids and liquids that intermolecular forces do not prevent gases from being compressed. 2. Why must heat be removed to change liquid water to ice? In order for freezing to occur, the motion of molecules must be slowed sufficiently for liquid particles to interact and become solids in a crystal structure, requiring the loss of heat energy. 3. How does air inside a balloon hold the rubber out? That is, what causes pressure? The collisions of gas particles with the walls of the balloon cause pressure (a force per unit area), resulting in a “blown up” balloon. 4. The air pressure inside an automobile tire increases when the tire becomes warm. Why is this so? The friction created when the tire is in contact with the road causes the temperature of the tire to increase, resulting in faster moving particles of gas which collide more with the tire walls generating a greater tire pressure. www.pembinatrails.ca/shaftesbury/mrdeakin adeakin@pembinatrails.ca (204) 888-5898 Shaftesbury High School, 2240 Grant Ave, Wpg, MB, R3P 0P7