Order of Magnitude Calculations for an Electron Ionization Source
advertisement
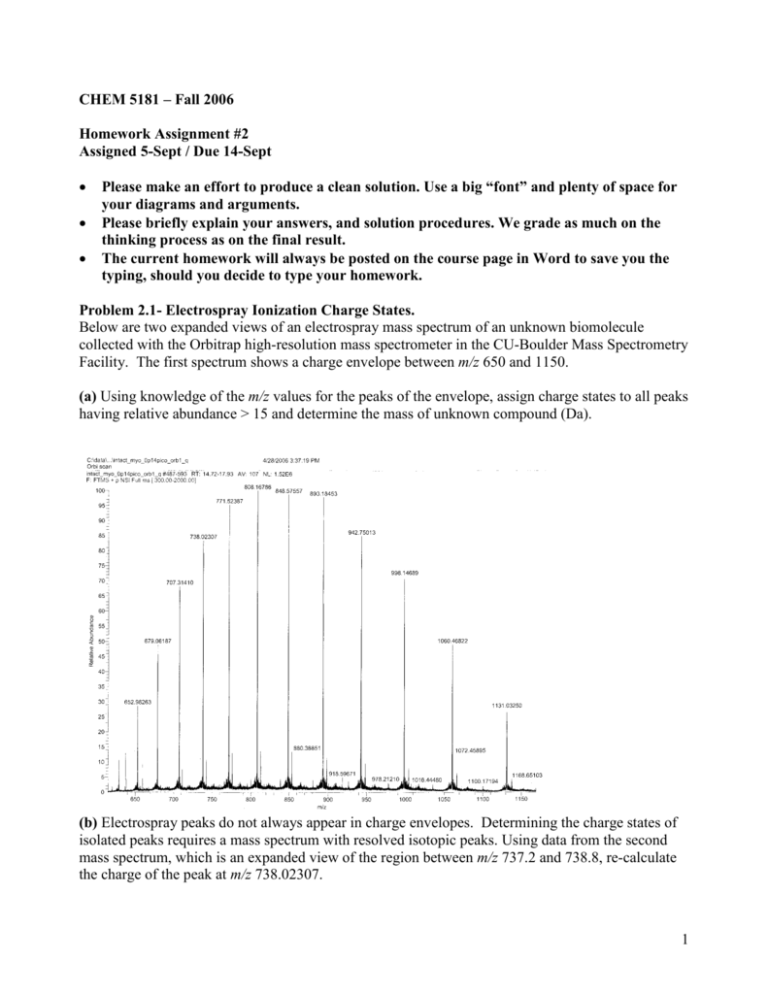
CHEM 5181 – Fall 2006 Homework Assignment #2 Assigned 5-Sept / Due 14-Sept Please make an effort to produce a clean solution. Use a big “font” and plenty of space for your diagrams and arguments. Please briefly explain your answers, and solution procedures. We grade as much on the thinking process as on the final result. The current homework will always be posted on the course page in Word to save you the typing, should you decide to type your homework. Problem 2.1- Electrospray Ionization Charge States. Below are two expanded views of an electrospray mass spectrum of an unknown biomolecule collected with the Orbitrap high-resolution mass spectrometer in the CU-Boulder Mass Spectrometry Facility. The first spectrum shows a charge envelope between m/z 650 and 1150. (a) Using knowledge of the m/z values for the peaks of the envelope, assign charge states to all peaks having relative abundance > 15 and determine the mass of unknown compound (Da). (b) Electrospray peaks do not always appear in charge envelopes. Determining the charge states of isolated peaks requires a mass spectrum with resolved isotopic peaks. Using data from the second mass spectrum, which is an expanded view of the region between m/z 737.2 and 738.8, re-calculate the charge of the peak at m/z 738.02307. 1 Problem 2.2- Ion Source Optics. The figure below is a schematic of an electron ionization source and its extraction optics. (FYI: this is the ion source used in the Aerosol Mass Spectrometer used by the Jimenez Lab). Ions are created inside the box to the left, as electrons are emitted from the filament (small circle below the “cathode” voltage) they ionize some of the neutral molecules inside the box. Using the values of voltages from the table below plot the velocity and acceleration vs. position curves for (a) an H2O+ ion and (b) a Hg+ ion as they are extracted by the ion optics and they enter the quadrupole mass analyzer (labeled as “rod system”). Assume that the entire box interior is sitting at the V1 (“ion reference”) voltage and that ions are formed on the central axis of the ion source. Measure the distances from the graph (please use mm and not inches) and assume that the graph is drawn on a 2:1 scale (twice as big as real). Note: Disregard superscripts on the voltages. 2 Problem 2.3: Processes that control unimolecular ion decomposition. As we discussed in lecture 2, the two main processes that control the fragmentation of an isolated unimolecular ion (under vacuum) once it has been formed (by any ionization technique) are the amount of energy deposited in the molecular ion and the chemical nature of the molecule (including its resistance to fragmentation). Here you have to calculate what would be the observed mass spectrum for acrolein and toluene using the breakdown curves given below for both molecular ions, and the ionization energy distributions shown in the figure in slide 25 of the notes (labeled Fig 14.29) for field ionization (FI), 70 eV electron ionization (EI) and chemical ionization (CE). Assume that the 0 in those three curves corresponds to the ionization potential of each species. The ionization potential of acrolein in 10.10 eV and that of benzene is 9.25 eV. Discretize all curves on 0.25 eV intervals (e.g. in Excel). For acrolein use the experimental data (symbols) rather than the model (lines). Note FYI: the breakdown curve of acrolein was obtained by photoionization with photons of increasing energy (using a tunable laser). The BC of benzene was obtained by performing chemical ionization with a series of reactants, each of which deposits a precise amount of energy on the molecular ion. Breakdown curve of the acrolein molecular ion (CH2CHCHO.+). (From Yue Li and Tomas Baer. The dissociation dynamics and thermochemistry of the acrolein ion studied by threshold photoelectron– photoion coincidence spectroscopy. International Journal of Mass Spectrometry Volume 218, Issue 1, 15 June 2002, Pages 37-48. http://dx.doi.org/10.1016/S1387-3806(02)00644-9) 3 Breakdown curve of the benzene molecular ion (from http://uigelz.ece.uiuc.edu/pub/presentations/rajesh_gec99.pdf) Problem 2.4- Order of Magnitude Calculations for an Electron Ionization Source Note: these calculations are approximate by nature, so don’t try to get many significant figures. Make the simplifications and approximations you need to get to a numerical answer. I am most interested in your getting the order of magnitude right for each step (i.e. within a factor of 2 or 3); and critically evaluating whether that order of magnitude makes sense in the bigger picture. If you are missing a number, estimate it (and make clear that you are doing so and how). Also, some of the later steps can be done even if you can’t do the earlier ones, so try each step independently. Please explain how you are trying to solve each step, so that I can give you partial credit even if you don’t get the final answer right. 4 (a) In a typical EI ion source, an emission current of 2.5 mA is used. What is the electron flux (electrons / s ) on this source? What is the speed of the electrons if their energy is 70 eV? What is the associated wavelength? Can you explain why 70 eV electrons are most effective at ionizing almost any molecule? (as compared with electrons with different kinetic energies). Electron path Effective Ionization Volume Filament (b) Assume that the actual ionization volume is a cube of 3 mm side, and that 100% of the source pressure of 10-8 Torr is due to N2. What is the ion current detected by the mass spectrometer? Assume that ions formed in the ionization volume are transmitted to the detector with 30% efficiency. Note that the EI cross section of N2 is 2.7 A2 (1A = 10-10 m). Refer to the course notes for the interpretation of the EI cross section. (c) What is the probability that a given electron that leaves the filament actually ionizes a water molecule? (d) What is the probability that a molecule that crosses the ionizer volume at its thermal speed only once (at 300K) is ionized? (this is the “ionization efficiency”). 5











